filmov
tv
Chemical Thermodynamics 3.6 - Adiabatic Processes

Показать описание
Short physical chemistry lecture on reversible adiabatic expansion and compression of ideal gases.
Adiabatic processes are those which occur without any heat. The relationship between final and initial temperature and volume in an adiabatic process depends on the constant volume heat capacity, which varies depending on the type of molecule in an ideal gas.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
Adiabatic processes are those which occur without any heat. The relationship between final and initial temperature and volume in an adiabatic process depends on the constant volume heat capacity, which varies depending on the type of molecule in an ideal gas.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
Chemical Thermodynamics 3.7 - Adiabatic / Isothermal Comparison
Chemical Thermodynamics: Lecture 3 - Comparison of Isothermal & Adiabatic Processes | Problem So...
Adiabatic Process - Work, Heat & Internal Energy, Gamma Ratio, Thermodynamics & Physics
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
Thermodynamic Processes (Animation)
Chemical thermodynamics|6 |Isothermal|Adiabatic|Expansion|Workheatenthalpy|Ideal gas |BScchemistry|
Calculate Work for Reversible and Irreversible Expansion/Compression
WORK IN AN ADIABATIC PROCESS | PV^γ | CHEMICAL THERMODYNAMICS | ECHO SERIES | PART-6
Chemical thermodynamics|7 |Isothermal|Adiabatic|Workheatenthalpy|van der Waals gas |BScchemistry|
Class 11 Chapter 6 | Thermodynamics 04 | Work done in Isothermal and Adiabatic Expansion of Gas |
Adiabatic Compression (Example)
Class 11 Chapter 6 | Thermodynamics 03 | Work Done by a Gas | Work Done on a Gs | IIT JEE / NEET |
Thermodynamics 06 || Isothermal and Adiabatic Process With Best Numericals JEE MAINS/NEET
The First Law of Thermodynamics: Internal Energy, Heat, and Work
thermodynamics Questions 5 | cyclic process #chemistry #jee #neet #short #youtubeshorts #shortvideo
Thermodynamics 03 || Heat : Specific heat Capacities Of Gases : Cp and Cv JEE MAINS/NEET||
Reversible Adiabatic Expansion. #thermodynamics #reversible #adiabatic #specificheatcapacity
Trick For Spontaneous Reaction In Thermodynamics #shorts
PV Diagrams, How To Calculate The Work Done By a Gas, Thermodynamics & Physics
Adiabatic Process ( Classroom Demonstration)
Enthalpy | Thermodynamics
Thermodynamics class 11 all formulas // Thermodynamics physics and chemistry //#viral #trending #pw
Beauty of the Brain😍 IQ - IIT Bombay
L-6 Thermodynamics - Adiabatic Process | IChO 2022 | Physical Chemistry | Rahul Mishra
Комментарии
 0:05:57
0:05:57
 0:22:46
0:22:46
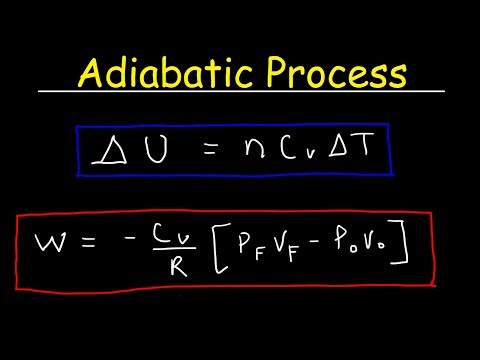 0:10:38
0:10:38
 0:11:27
0:11:27
 0:09:19
0:09:19
 0:24:21
0:24:21
 0:04:39
0:04:39
 0:18:44
0:18:44
 0:06:44
0:06:44
 0:43:04
0:43:04
 0:06:08
0:06:08
 0:40:08
0:40:08
 1:44:44
1:44:44
 0:05:44
0:05:44
 0:00:53
0:00:53
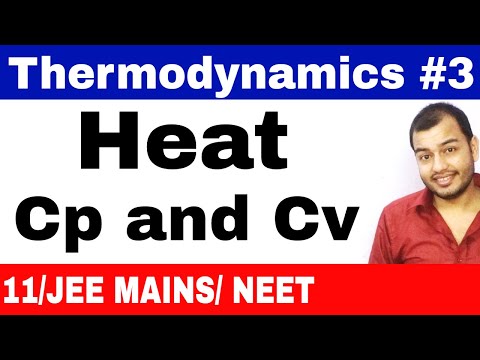 0:34:56
0:34:56
 0:00:46
0:00:46
 0:00:33
0:00:33
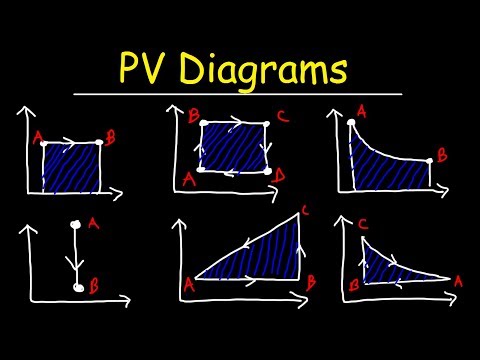 0:20:17
0:20:17
 0:03:39
0:03:39
 0:10:55
0:10:55
 0:00:08
0:00:08
 0:00:19
0:00:19
 0:53:21
0:53:21