filmov
tv
Bragg's Law | Physics

Показать описание
Suppose, and X-ray bean is incident on a solid, making an angle θ with the planes of the atoms. These X-rays are diffracted by different atoms and the diffracted rays interfere. In certain directions, the interference is constructive and we obtain strong reflected X-rays. The analysis shows that there will be a strong reflected X-ray beam only if
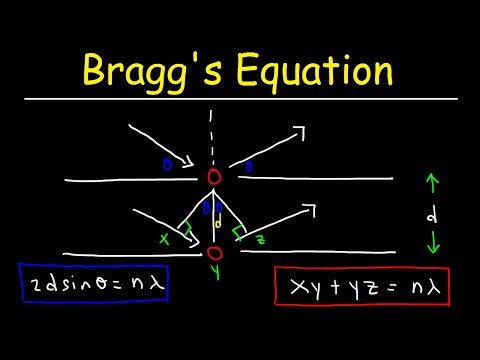
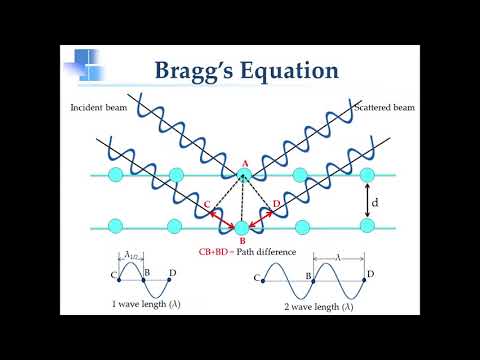
2dsinθ=nλ
where n is an integer. this equation is known as Bragg's law.
Example: X-rays are known to be electromagnetic radiation with wavelength of the order of 1A
∘
. They are produced when accelerated electrons strike target inside an evacuated tube. It is well known that when an electron is accelerated through a potential difference of V it acquires energy eV. If all this energy is used in producing one quantum of X-radiation, then hf=eV. It is likely that the electron may have lost some of its acquired energy before producing the quantum of radiation. The f gives the maximum possible frequency of X-radiation emitted and that corresponds to the short wavelength limit of the emitted spectrum. In general, therefore, X-ray spectra consist of a continuous spectrum upon which is superposed a line spectrum that is characteristic of the element used as target. For some time after the discovery of X-rays, there was considerable speculation about the nature of X-rays. Max Von Laue found, in 1912 that if X-rays are passed through a crystal they get diffracted. As Laue
patterns are difficult to interpret, Bragg worked out a simple equation that predicts the conditions under which diffracted X-rays beams from a crystal are possible. In its simplest form, Bragg's Law is given by λ=2dsinθ where d is the perpendicular distance between the planes contining atoms and θ is the glancing angle at which the X-rays fall on the crystal. λ is known, the distance may be found from experimental measurements. This is the basis for the field of X-ray crystallography in which the structure of crystals is determined by using X-rays. Find the photon is the order of energy associated with an x-ray.
2dsinθ=nλ
where n is an integer. this equation is known as Bragg's law.
Example: X-rays are known to be electromagnetic radiation with wavelength of the order of 1A
∘
. They are produced when accelerated electrons strike target inside an evacuated tube. It is well known that when an electron is accelerated through a potential difference of V it acquires energy eV. If all this energy is used in producing one quantum of X-radiation, then hf=eV. It is likely that the electron may have lost some of its acquired energy before producing the quantum of radiation. The f gives the maximum possible frequency of X-radiation emitted and that corresponds to the short wavelength limit of the emitted spectrum. In general, therefore, X-ray spectra consist of a continuous spectrum upon which is superposed a line spectrum that is characteristic of the element used as target. For some time after the discovery of X-rays, there was considerable speculation about the nature of X-rays. Max Von Laue found, in 1912 that if X-rays are passed through a crystal they get diffracted. As Laue
patterns are difficult to interpret, Bragg worked out a simple equation that predicts the conditions under which diffracted X-rays beams from a crystal are possible. In its simplest form, Bragg's Law is given by λ=2dsinθ where d is the perpendicular distance between the planes contining atoms and θ is the glancing angle at which the X-rays fall on the crystal. λ is known, the distance may be found from experimental measurements. This is the basis for the field of X-ray crystallography in which the structure of crystals is determined by using X-rays. Find the photon is the order of energy associated with an x-ray.
Комментарии
 0:04:08
0:04:08
 0:14:59
0:14:59
 0:00:40
0:00:40
 0:05:47
0:05:47
 0:05:33
0:05:33
 0:08:36
0:08:36
 0:12:09
0:12:09
 0:00:12
0:00:12
 0:05:13
0:05:13
 0:25:46
0:25:46
 0:17:52
0:17:52
 0:06:55
0:06:55
 0:01:00
0:01:00
 0:18:45
0:18:45
 0:00:06
0:00:06
 0:16:29
0:16:29
 0:14:02
0:14:02
 0:03:46
0:03:46
 0:06:47
0:06:47
 0:06:36
0:06:36
 0:02:54
0:02:54
 0:16:51
0:16:51
 0:00:30
0:00:30
 0:04:41
0:04:41