filmov
tv
Genetic Knock-Down (e.g. with RNAi - siRNA or shRNA) & Knock-Out (e.g. with CRISPR/Cas)

Показать описание
Genetic knock-down versus knock-out and a few different ways to bring it about. If you want to *interfere* with protein production without messing with the gene, you can turn to RNA interference to lessen its protein. But if instead you want to permanently knock the gene out, CRISPR/Cas9 offers you a route.
If you walk into a restaurant at 6am you might get a breakfast menu whereas at 6pm you’d get a dinner one. It’s not that the restaurant doesn’t magically stop knowing how to make a pancake when the clock strikes noon (and if you ask nicely they might still make you one). But they assume people won’t want pancakes at night.
Similarly, your cells produce different proteins at different times but they still have the genetic recipes for making the proteins that aren’t “on the menu” at some point in time - unless you destroy the original recipes, which CRISPR/Cas9 can do, but RNAi can’t. All the original recipes (genes) are written in DNA and locked up in a membrane-bound compartment called the nucleus. To go “on the menu” they have to be transcribed - copied into messenger RNA (mRNA) form and taken into the cytoplasm (general cell area) where the chefs are - it’s this mRNA that RNAi destroys.
As a result, knocking down a gene (such as with RNAi) is like taking something off the menu whereas knocking out a gene (such as with CRISPR/Cas9 genome editing) is destroying the original recipe so the restaurant doesn’t know how to make it anymore. No more pancakes. Ever.
The 2 methods do have other things in common however (In addition to lowering the effects of specific genes). They both achieve their specificity through the use of RNA guides. RNA & DNA letters can base pair with each other - like matching puzzles pieces “A” binds “T” (in DNA) or “U” (in RNA) and “C” binds “G.” So you can get 2 complementary sequences to bind to each other and a sequence can act as a guide to direct silencing machinery to a complementary sequence.
Let’s start with knock-down (via RNAi)…
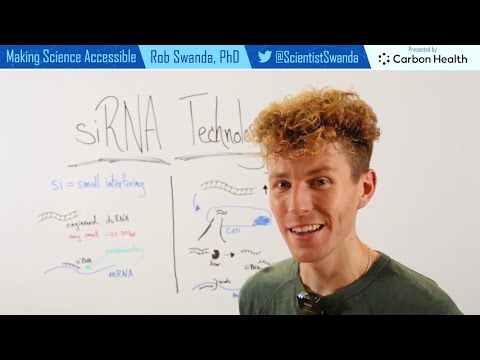
RNAi relies on small RNA (sRNA) guides. sRNAs are small (only ~22 nucleotides (nt) (RNA letters) long) but mighty! Their sequences “match” sequences in specific mRNAs (protein recipes), so they’re able to bind those mRNAs. And they bring mRNA-destruction machinery with them (before they bind the targets they’re loaded into a protein called Argonaute (Ago) which can shut down some targets on its own and call for backup as needed.
This is a natural mechanism your cells use to take things off the menu (without our intervention). But they use a form of RNAi that uses microRNAs (miRNAs) which are less specific than the ones we use in the lab, but can target multiple related genes (kinda like being able to take all the breakfast-only items off the menu when it’s lunch time).
Our cells use miRNA to regulate over half of all our genes. miRNAs are made from special genes that get processed differently than protein-making genes. And they get loaded into a Ago and guide it to target mRNAs. If the miRNA and target mRNA match completely, Ago can cut it (act as an endonuclease)*. But usually miRNA only match partially - it’s enough to have full complementarity of a 6-8 letter long “seed sequence” - this will keep Ago on there while it calls for backup to help it degrade the target.
*at least our main Ago, Ago2 can - it’s the only** one of the 4 human versions of Ago that can slice
**it was thought this for a long time but recently Ago3 was found to be able to slice in certain circumstances, but using shorter guides
The specific but not *too specific*-ness of miRNA makes it great for targeting related things at the same time, so it’s great for its natural purpose but not great if you want to use RNAi as a tool to artificially knock down specific genes. In that case you want something more specific. You can get higher specificity by introducing guides that are fully complementary so less likely to match multiple things.
And these fully complementary guides also enable Ago to slice (cut) the target, directly harming it, rather than “just” calling in other proteins (cofactors) to help with the destruction.
You can introduce the “guide-to-be” to cells in a dish by transfecting them - sticking in foreign stuff - with double-stranded RNA (dsRNA) that the cells can process into mature guide small interfering RNA (siRNA), or siRNA duplexes themselves. Or you can infect them with a virus (transduce them) that sticks in a circle of DNA called a vector that the cells can transcribe into a short hairpin RNA (shRNA) that can get processed into mature guide. It’s kinda like “give a man a fish” (siRNA duplex) vs. “teach a man to fish” (shRNA) depending on your wants/needs.
Finished in comments
If you walk into a restaurant at 6am you might get a breakfast menu whereas at 6pm you’d get a dinner one. It’s not that the restaurant doesn’t magically stop knowing how to make a pancake when the clock strikes noon (and if you ask nicely they might still make you one). But they assume people won’t want pancakes at night.
Similarly, your cells produce different proteins at different times but they still have the genetic recipes for making the proteins that aren’t “on the menu” at some point in time - unless you destroy the original recipes, which CRISPR/Cas9 can do, but RNAi can’t. All the original recipes (genes) are written in DNA and locked up in a membrane-bound compartment called the nucleus. To go “on the menu” they have to be transcribed - copied into messenger RNA (mRNA) form and taken into the cytoplasm (general cell area) where the chefs are - it’s this mRNA that RNAi destroys.
As a result, knocking down a gene (such as with RNAi) is like taking something off the menu whereas knocking out a gene (such as with CRISPR/Cas9 genome editing) is destroying the original recipe so the restaurant doesn’t know how to make it anymore. No more pancakes. Ever.
The 2 methods do have other things in common however (In addition to lowering the effects of specific genes). They both achieve their specificity through the use of RNA guides. RNA & DNA letters can base pair with each other - like matching puzzles pieces “A” binds “T” (in DNA) or “U” (in RNA) and “C” binds “G.” So you can get 2 complementary sequences to bind to each other and a sequence can act as a guide to direct silencing machinery to a complementary sequence.
Let’s start with knock-down (via RNAi)…
RNAi relies on small RNA (sRNA) guides. sRNAs are small (only ~22 nucleotides (nt) (RNA letters) long) but mighty! Their sequences “match” sequences in specific mRNAs (protein recipes), so they’re able to bind those mRNAs. And they bring mRNA-destruction machinery with them (before they bind the targets they’re loaded into a protein called Argonaute (Ago) which can shut down some targets on its own and call for backup as needed.
This is a natural mechanism your cells use to take things off the menu (without our intervention). But they use a form of RNAi that uses microRNAs (miRNAs) which are less specific than the ones we use in the lab, but can target multiple related genes (kinda like being able to take all the breakfast-only items off the menu when it’s lunch time).
Our cells use miRNA to regulate over half of all our genes. miRNAs are made from special genes that get processed differently than protein-making genes. And they get loaded into a Ago and guide it to target mRNAs. If the miRNA and target mRNA match completely, Ago can cut it (act as an endonuclease)*. But usually miRNA only match partially - it’s enough to have full complementarity of a 6-8 letter long “seed sequence” - this will keep Ago on there while it calls for backup to help it degrade the target.
*at least our main Ago, Ago2 can - it’s the only** one of the 4 human versions of Ago that can slice
**it was thought this for a long time but recently Ago3 was found to be able to slice in certain circumstances, but using shorter guides
The specific but not *too specific*-ness of miRNA makes it great for targeting related things at the same time, so it’s great for its natural purpose but not great if you want to use RNAi as a tool to artificially knock down specific genes. In that case you want something more specific. You can get higher specificity by introducing guides that are fully complementary so less likely to match multiple things.
And these fully complementary guides also enable Ago to slice (cut) the target, directly harming it, rather than “just” calling in other proteins (cofactors) to help with the destruction.
You can introduce the “guide-to-be” to cells in a dish by transfecting them - sticking in foreign stuff - with double-stranded RNA (dsRNA) that the cells can process into mature guide small interfering RNA (siRNA), or siRNA duplexes themselves. Or you can infect them with a virus (transduce them) that sticks in a circle of DNA called a vector that the cells can transcribe into a short hairpin RNA (shRNA) that can get processed into mature guide. It’s kinda like “give a man a fish” (siRNA duplex) vs. “teach a man to fish” (shRNA) depending on your wants/needs.
Finished in comments
Комментарии
 0:28:55
0:28:55
 0:07:31
0:07:31
 0:25:59
0:25:59
 0:01:21
0:01:21
 0:04:26
0:04:26
 0:03:52
0:03:52
 0:09:06
0:09:06
 0:01:51
0:01:51
 0:01:57
0:01:57
 0:05:57
0:05:57
 0:03:31
0:03:31
 0:03:32
0:03:32
 0:02:01
0:02:01
 0:00:21
0:00:21
 0:02:01
0:02:01
 0:01:39
0:01:39
 0:29:42
0:29:42
 0:16:18
0:16:18
 0:20:22
0:20:22
 0:01:46
0:01:46
 0:14:28
0:14:28
 0:06:20
0:06:20
 0:01:52
0:01:52
 0:55:16
0:55:16