filmov
tv
BOYLE'S LAW | Animation

Показать описание
This time we are going to talk about “Boyle’s Law”.
In a gas, its physical behavior is described by these four variables namely: Pressure, Volume, Temperature, and Amount (mol). In this video, we will have Boyle’s Law which is concerning the relationship of pressure and volume.
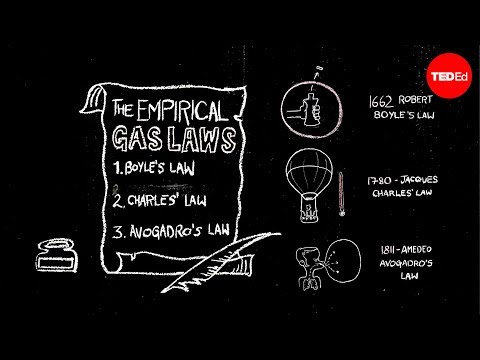
Boyle’s law was formulated by the famous scientist Robert Boyle in 1662. This law relates to the expansion and compression of the gas at a constant temperature.
In his experiment, he has a J-shaped tube with the shorter end close and the long end open. He put mercury in the tube and determined the volume of trapped gas. When he pours some more mercury, he found out that the volume of trapped gas decreases while the change in height of the mercury increases, which indicates the pressure also increases.
Find out more by watching the video.
*Enjoy this fun and informative video from EarthPen
#BoylesLaw #Chemistry #EducationalVideo
CONTACT US
In a gas, its physical behavior is described by these four variables namely: Pressure, Volume, Temperature, and Amount (mol). In this video, we will have Boyle’s Law which is concerning the relationship of pressure and volume.
Boyle’s law was formulated by the famous scientist Robert Boyle in 1662. This law relates to the expansion and compression of the gas at a constant temperature.
In his experiment, he has a J-shaped tube with the shorter end close and the long end open. He put mercury in the tube and determined the volume of trapped gas. When he pours some more mercury, he found out that the volume of trapped gas decreases while the change in height of the mercury increases, which indicates the pressure also increases.
Find out more by watching the video.
*Enjoy this fun and informative video from EarthPen
#BoylesLaw #Chemistry #EducationalVideo
CONTACT US
Комментарии
 0:02:53
0:02:53
 0:02:02
0:02:02
 0:01:36
0:01:36
 0:02:08
0:02:08
 0:05:30
0:05:30
 0:00:26
0:00:26
 0:02:50
0:02:50
 0:01:22
0:01:22
 0:08:59
0:08:59
 0:08:19
0:08:19
 0:03:21
0:03:21
 0:02:03
0:02:03
 0:00:21
0:00:21
 0:00:21
0:00:21
 0:01:15
0:01:15
 0:00:25
0:00:25
 0:01:09
0:01:09
 0:02:34
0:02:34
 0:00:21
0:00:21
 0:00:16
0:00:16
 0:00:15
0:00:15
 0:00:46
0:00:46
 0:03:55
0:03:55
 0:04:33
0:04:33