filmov
tv
Boyle's Law | Experiment | Graphical Representation | Examples | Whiteboard Animation | Explained

Показать описание
In this fully Animated lecture you will learn about Derivation and Graphical Explanation of Boyle's Law.
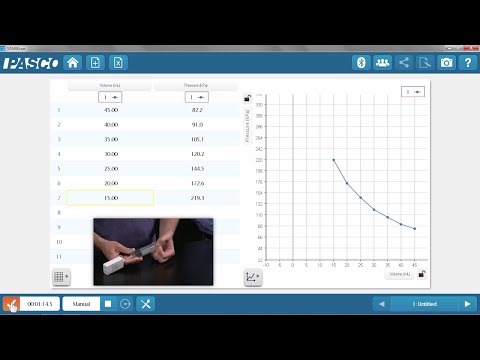
According to Boyle's Law.
The volume of a given mass of a gas, is inversely proportional to its pressure. as long as temperature remains constant.
Mathematically it can be written as, v is inversely proportional to P, while Temperature T, becomes constant. To change the proportionality sign into an equal sign, we need to add a constant, which we'll call k this equation can also be written as P V is equal to k.
k is a proportionality constant. The value of k is the same, for the same amount of a given gas. Therefore Boyle's law can be stated as a product of, pressure and volume of a fixed mass of a gas, is constant at, constant temperature.
Let me teach you, a common application of Boyle's Law.
Boyle's law helps us to understand, how changes in volume and pressure in our lungs, during inhalation and exhalation.
When we inhale, lungs expand, that increases the volume of our chest cavity. that decreases the pressure, causing air to rush in to the lungs
On the other hand, during exhalation lungs Contract, that decreases the volume of our chest cavity. That results in an increase in pressure inside the lungs, forcing air out.
So according to Boyle's law, when volume increases, the pressure decreases. and when there is a decrease in volume, pressure increases.
#boyleslaw
#boyle
#gas
#gaslaws
#education
#respiration
#chemistry
#sciencefacts
#science
#physics
Please subscribe our channel for more informative videos...
@afzaalchemist
According to Boyle's Law.
The volume of a given mass of a gas, is inversely proportional to its pressure. as long as temperature remains constant.
Mathematically it can be written as, v is inversely proportional to P, while Temperature T, becomes constant. To change the proportionality sign into an equal sign, we need to add a constant, which we'll call k this equation can also be written as P V is equal to k.
k is a proportionality constant. The value of k is the same, for the same amount of a given gas. Therefore Boyle's law can be stated as a product of, pressure and volume of a fixed mass of a gas, is constant at, constant temperature.
Let me teach you, a common application of Boyle's Law.
Boyle's law helps us to understand, how changes in volume and pressure in our lungs, during inhalation and exhalation.
When we inhale, lungs expand, that increases the volume of our chest cavity. that decreases the pressure, causing air to rush in to the lungs
On the other hand, during exhalation lungs Contract, that decreases the volume of our chest cavity. That results in an increase in pressure inside the lungs, forcing air out.
So according to Boyle's law, when volume increases, the pressure decreases. and when there is a decrease in volume, pressure increases.
#boyleslaw
#boyle
#gas
#gaslaws
#education
#respiration
#chemistry
#sciencefacts
#science
#physics
Please subscribe our channel for more informative videos...
@afzaalchemist
Комментарии
 0:02:09
0:02:09
 0:04:27
0:04:27
 0:01:45
0:01:45
 0:04:33
0:04:33
 0:04:30
0:04:30
 0:03:13
0:03:13
 0:02:53
0:02:53
 0:05:01
0:05:01
 0:00:26
0:00:26
 0:03:10
0:03:10
 0:08:58
0:08:58
 0:03:55
0:03:55
 0:00:45
0:00:45
 0:01:22
0:01:22
 0:07:18
0:07:18
 0:00:32
0:00:32
 0:01:00
0:01:00
 0:04:18
0:04:18
 0:03:06
0:03:06
 0:05:13
0:05:13
 0:05:26
0:05:26
 0:01:15
0:01:15
 0:05:38
0:05:38
 0:02:40
0:02:40