filmov
tv
Given atomic number find position and vice-versa (Solved example) | Chemistry | Khan Academy
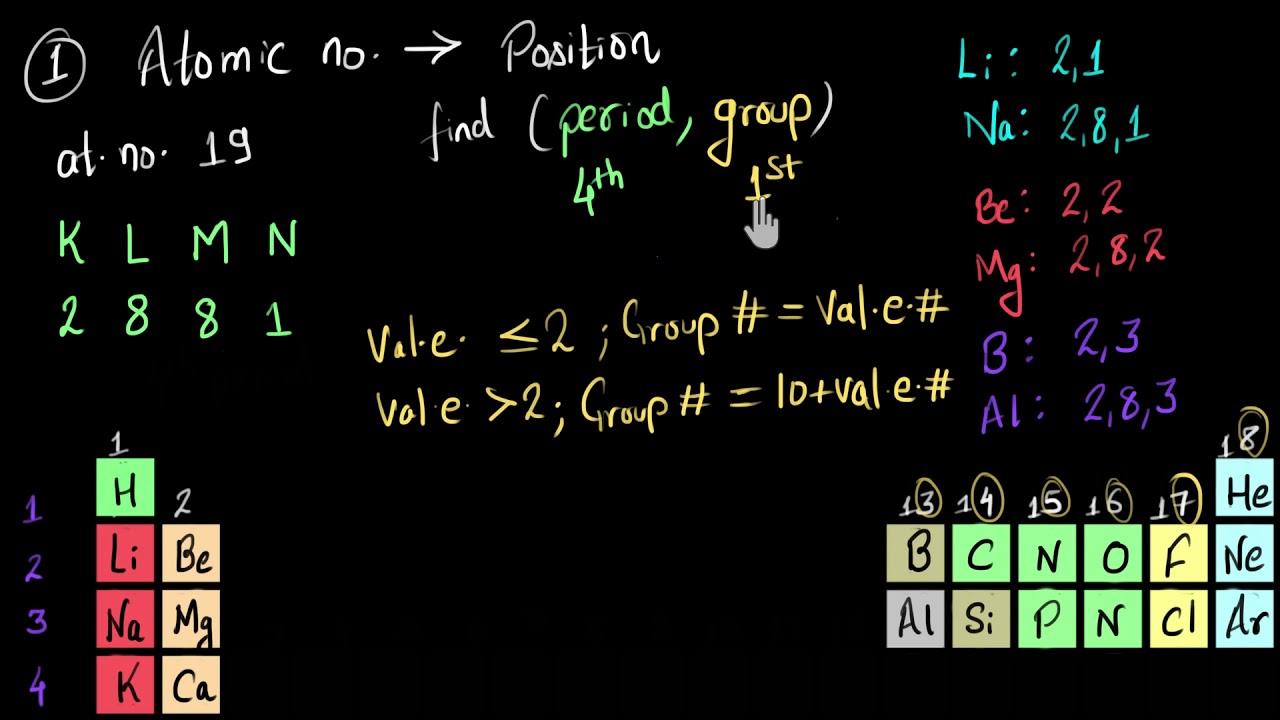
Показать описание
If you are given with the atomic number of an element you can find it's period number and group number. The period number is related to the number of electron occupied shells in the element and the period number is linked to its valence electrons. Let's see how.
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Created by Ram Prakash
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Created by Ram Prakash
Given atomic number find position and vice-versa (Solved example) | Chemistry | Khan Academy
How to find Group, Period and Block of an element?
Trick To Find 'GROUP & PERIOD' Number Of Any Element | Chemistry Tricks
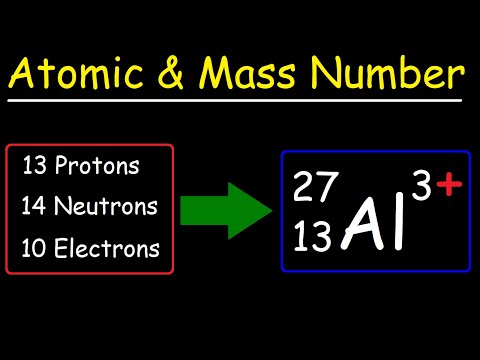
Atomic Number, Mass Number, and Net Electric Charge
Given atomic number find position and vice versa Solved example | Chemistry | Khan Academy Urdu
Electron Configuration - How To Identify The Element
How To Calculate The Number of Protons, Neutrons, and Electrons - Chemistry
Easy tricks to identify the Group, Block and Period of an element by using Electronic Configuration.
LAST MOMENT SUGGESTIONS | ICSE | PHYSICAL SCIENCE | 6-CHAPTERS IN ONE SHOT | MARIA'S VIRTUAL CL...
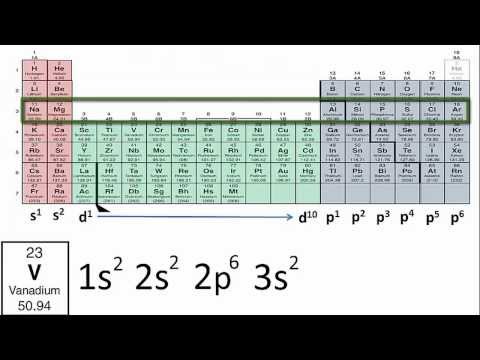
Writing Electron Configurations Using Only the Periodic Table
Best Trick to find Maximum no. of Elements in a Period #reels #shorts #cbse
Trick to find group number and period number in Periodic table/periodic table tricks/class 12 chem
How To Determine The 4 Quantum Numbers From an Element or a Valence Electron
Finding the Position in Periodic Table (by atomic number)
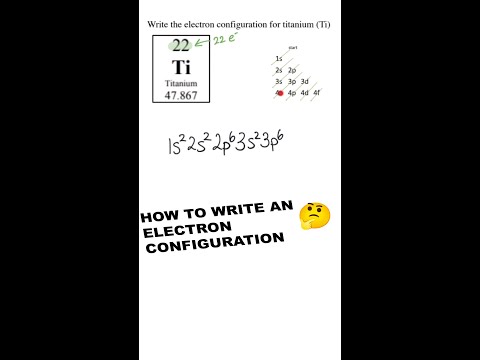
How to Write an Electron Configuration #chemistry #shorts #science #education #homework
##Trick to find the position of elements in the periodic table in 30 sec##Best method👍👍
Predict the position of element having atomic number 72 (Period number and group number)
How to find the position of elements in periodic table?
Find Atomic number of any Element in few seconds | How to Find Atomic Number | Atomic Number Trick |
How to find the Protons Neutrons and Electrons of an element on the Periodic table
11 chap 3 | Periodic Table 03 || How to Find Group, Period and Block of any Element || spdf trick
Worked example: Identifying an element from successive ionization energies | Khan Academy
Finding Protons, Electron, Neutrons | Chemistry Class 9 / 10 Science | YouTube Shorts by JP Sir
118 Elements name in 39 Sec | Basic ke bache #Varanasi | #Nmms
Комментарии
 0:11:08
0:11:08
 0:08:27
0:08:27
 0:04:03
0:04:03
 0:11:41
0:11:41
 0:08:26
0:08:26
 0:06:11
0:06:11
 0:13:12
0:13:12
 0:12:31
0:12:31
 1:33:26
1:33:26
 0:04:52
0:04:52
 0:00:51
0:00:51
 0:11:10
0:11:10
 0:04:25
0:04:25
 0:13:21
0:13:21
 0:01:00
0:01:00
 0:04:56
0:04:56
 0:02:11
0:02:11
 0:07:39
0:07:39
 0:13:59
0:13:59
 0:04:23
0:04:23
 0:31:22
0:31:22
 0:02:31
0:02:31
 0:00:26
0:00:26
 0:00:16
0:00:16