filmov
tv
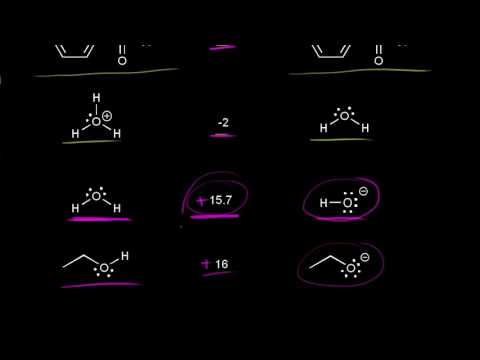
What Makes A Good Leaving Group?

Показать описание
What makes a good leaving group? They are weak bases. How to rank leaving group ability. How to identify leaving groups.
If you found this video useful, please check out our membership benefits. MOC members get access to over 1500 quizzes on Good Leaving Groups and other topics, plus Flashcards, the Reaction Guide, and more.
If you found this video useful, please check out our membership benefits. MOC members get access to over 1500 quizzes on Good Leaving Groups and other topics, plus Flashcards, the Reaction Guide, and more.
What Makes A Good Leaving Group?
Leaving Group Stability - SN1 and SN2 Reactions
How to Make OH into a Good Leaving Group
18.03 What Makes a Good Leaving Group?
Nucleophiles, Electrophiles, Leaving Groups, and the SN2 Reaction
Leaving Groups in Substitution and Elimination Reactions (vid 1 of 2) by Leah4sci
We Are Leaving - Eddie Brock ('Venom') Edit | Rather Be - Clean Bandit ft Jess Glynne
Sn1 and Sn2: leaving group
SECRET to being more LIKEABLE ( Leaving best first Impression ) 👍
Leaving Groups In Organic Chemistry | IIT JEE / NEET | Vineet Khatri | ATP STAR KOTA
What Trevor Noah Learned About America By Leaving America
How to Quit a Job: Leaving on Good Terms | Indeed
Effect of Leaving Groups on Sn2 Reactions
Why I Cried After Leaving North Korea #86
Leaving Group Derivatives
Why foreigners are leaving Japan
SN1 vs. SN2: The Nucleophile and Leaving Group
Harry Styles talks about Zayn Malik leaving the One Direction #shorts
Ash Ketchum Is LEAVING Pokemon! #shorts
Did Keenan Allen make a mistake leaving L.A.? ⚡️ #chargers #keenanallen #NFL
THIS Is The Moment Your Ex Will REGRET Leaving You
Squeeze a pimple without leaving a scar ?
Niklas Christl Is Leaving YouTube
Leaving Group Ability || Common, Super and Hyper Leaving Group 🔥🔥
Комментарии
 0:08:40
0:08:40
 0:12:17
0:12:17
 0:13:42
0:13:42
 0:09:12
0:09:12
 0:06:05
0:06:05
 0:08:46
0:08:46
 0:00:50
0:00:50
 0:06:52
0:06:52
 0:06:40
0:06:40
 0:17:44
0:17:44
 0:07:32
0:07:32
 0:03:26
0:03:26
 0:07:45
0:07:45
 0:01:00
0:01:00
 0:04:45
0:04:45
 0:00:55
0:00:55
 0:14:54
0:14:54
 0:00:57
0:00:57
 0:00:54
0:00:54
 0:00:56
0:00:56
 0:11:13
0:11:13
 0:00:09
0:00:09
 0:00:15
0:00:15
 0:28:45
0:28:45