filmov
tv
Phase diagrams of binary solutions: dew point and bubble point

Показать описание
In this video, prof. Márcio Neto shows how phase diagrams of binary solutions are constructed, and shows how to use them to obtain the dew point and bubble point of a given system.
Phase diagrams of binary solutions: dew point and bubble point
Binary Phase Diagrams Explained
Igneous Petrology Series: Lesson 5 - Binary solid solution phase diagrams
y-x Phase Diagram for VLE of a Binary Mixture
How to use phase diagrams and the lever rule to understand metal alloys
Physical Chemistry: Binary Phase Mixture and Phase Diagrams for Ideal Systems
noc18-mm20 Lecture 13-Phase Diagram formation:Binary Solution
Distillation and phase equilibria
Lecture 19 Binary Phase Diagrams Part 1
Phase Diagram 3: Binary solid solution
Phase Diagrams and Lever Rule example problem
2.1 | MSE104 - Binary Phase Diagrams
PHASE DIAGRAM (PART 4) : BINARY EUTECTIC PHASE DIAGRAM (LIMITED SOLID SOLUTION)
PCE58 Binary phase diagram of an ideal solution
T-X-Y DIAGRAM OF BINARY VLE MIXTURES
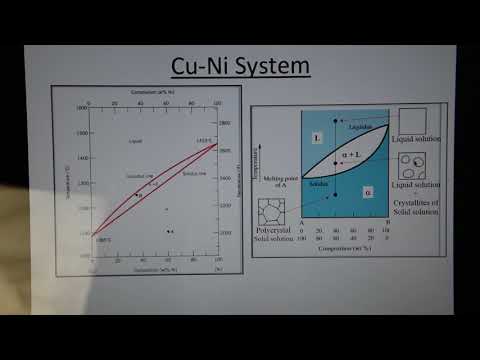
Binary Phase Diagrams - Cu-Ni System
Pressure-Composition Phase Diagram
Binary Phase Diagram (Txy and xy)
Txy and Pxy Diagrams
MEC281 : EXERCISE BINARY EUTECTIC PHASE DIAGRAM (NO SOLID SOLUTION)
Binary phase diagrams
Phase Diagrams 1 - Binary Eutectics
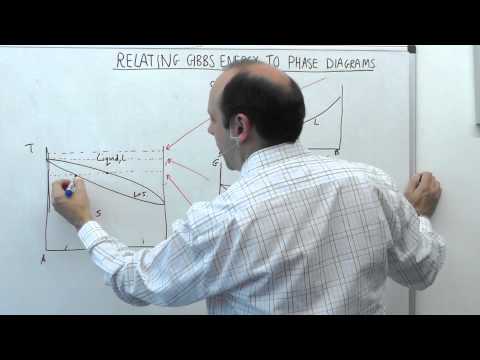
5.2 | MSE104 - Gibbs Energy Curves
Plagioclase Binary Phase Diagram
Комментарии
 0:06:37
0:06:37
 0:07:15
0:07:15
 0:06:37
0:06:37
 0:03:08
0:03:08
 0:23:25
0:23:25
 0:13:28
0:13:28
 0:29:19
0:29:19
 0:03:51
0:03:51
 0:20:23
0:20:23
 0:16:55
0:16:55
 0:05:00
0:05:00
 0:17:57
0:17:57
 0:20:52
0:20:52
 0:09:15
0:09:15
 0:08:08
0:08:08
 0:06:39
0:06:39
 0:13:08
0:13:08
 0:10:07
0:10:07
 0:14:53
0:14:53
 0:13:09
0:13:09
 0:05:16
0:05:16
 0:08:12
0:08:12
 0:26:14
0:26:14
 0:09:27
0:09:27