filmov
tv
5.1 | MSE104 - Thermodynamics of Solutions

Показать описание
Part 1 of lecture 5. Thermodynamics of solutions.
Enthalpy of mixing 4:56
Entropy of Mixing 24:14
Gibb's Energy of Mixing (The Regular Solution Model) 39:56
Lecturer: Dr David Dye.
Licence: Creative Commons
Department of Materials, Imperial College, London, UK
Enthalpy of mixing 4:56
Entropy of Mixing 24:14
Gibb's Energy of Mixing (The Regular Solution Model) 39:56
Lecturer: Dr David Dye.
Licence: Creative Commons
Department of Materials, Imperial College, London, UK
5.1 | MSE104 - Thermodynamics of Solutions
5.2 | MSE104 - Gibbs Energy Curves
9.1 | MSE104 Non-equilibrium cooling of steels
Chemical Thermodynamics 5.1 - Entropy Temp Dependence 1 (Old Version)
thermodynamics II - HW 1 - 5 solutions
1.1 | MSE104 - Introduction to Phases
EMA5001 L00-05 Kinetics and phase transformation vs Thermodynamics
Phase diagram thermodynamics versus kinetics
Thermodynamics Part 5 Calculating Gibbs Free Energy
problem 1-5 - Thermodynamics Sears W. Salinger - Solution Manual
Thermodynamics: Gaskell Problem 9.5
Thermodynamics - The enthalpies of solution - AQA A2 Chemistry - Unit 5 - 3.5.1
4.1 | MSE104 - Example Problems in Binary Eutectics
Surface Thermodynamics
thermodynamics part 1
Lecture 23: Building Binary Phase Diagrams, Part I
Thermodynamics: Gaskell Problem 9.4
Thermodynamics Problem Set #1-4
thermodynamics II - HW 1 - 7 solutions
Thermodynamics: Regular Solutions, Bonding, and the Competition between Enthalpy and Entropy
thermodynamics II - hw 1 - 3 solutions
thermodynamics II - hw 2-1 solutions
How To Calculate Entropy Changes: Mixing Ideal Gases
Ep11 Thermodynamics, ideal solutions, entropy - UC San Diego - NANO 134 Darren Lipomi
Комментарии
 0:48:08
0:48:08
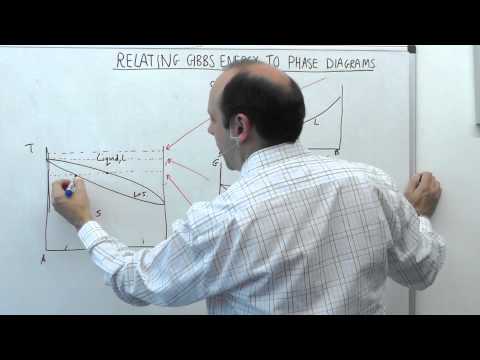 0:26:14
0:26:14
 0:28:47
0:28:47
 0:09:21
0:09:21
 0:16:58
0:16:58
 0:19:27
0:19:27
 0:13:45
0:13:45
 0:08:43
0:08:43
 0:15:11
0:15:11
 0:00:36
0:00:36
 0:05:41
0:05:41
 0:01:30
0:01:30
 0:32:12
0:32:12
 0:05:14
0:05:14
 1:18:49
1:18:49
 0:46:50
0:46:50
 0:09:50
0:09:50
 0:11:15
0:11:15
 0:10:32
0:10:32
 1:12:22
1:12:22
 0:12:27
0:12:27
 0:09:14
0:09:14
 0:04:20
0:04:20
 0:50:45
0:50:45