filmov
tv
How to Write the formula for Nitrogen Monoxide

Показать описание
In this video we'll write the correct formula for Nitrogen Monoxide.
To write the formula for Nitrogen Monoxide we’ll use the Periodic Table and follow some simple rules.
When we have a non-metal and a non-metal we have a molecular compound (sometimes called covalent). Molecular compounds are some of the simplest to name.
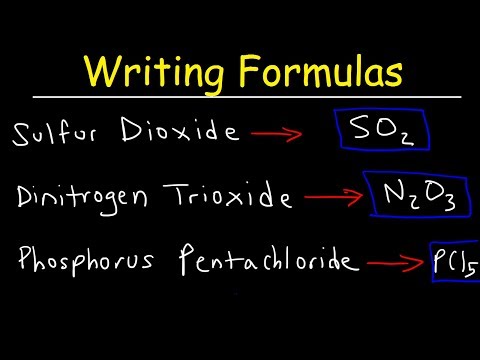
Keys for Writing the Formulas for Molecular Compounds:
- Write the element symbol for both elements.
- Place a subscript after each element according to its prefix.
Note: Don’t write the subscript '1'.
---------
Prefixes
mono- 1
di- 2
tri- 3
tetra- 4
penta- 5
hexa- 6
hepta- 7
octa- 8
nona- 9
deca- 10
---------
Caution: We only write "mono" for the second element in a molecular compound. So CO would be carbon monoxide. Monocarbon monooxide is incorrect.
For a complete tutorial on naming and formula writing visit:
or watch
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
To write the formula for Nitrogen Monoxide we’ll use the Periodic Table and follow some simple rules.
When we have a non-metal and a non-metal we have a molecular compound (sometimes called covalent). Molecular compounds are some of the simplest to name.
Keys for Writing the Formulas for Molecular Compounds:
- Write the element symbol for both elements.
- Place a subscript after each element according to its prefix.
Note: Don’t write the subscript '1'.
---------
Prefixes
mono- 1
di- 2
tri- 3
tetra- 4
penta- 5
hexa- 6
hepta- 7
octa- 8
nona- 9
deca- 10
---------
Caution: We only write "mono" for the second element in a molecular compound. So CO would be carbon monoxide. Monocarbon monooxide is incorrect.
For a complete tutorial on naming and formula writing visit:
or watch
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Writing Chemical Formulas For Ionic Compounds
Writing Ionic Formulas: Introduction
How to write an equation or formula in Word.
Excel How To Write Formulas
Writing Chemical Formulas For Covalent Molecular Compounds
How to Write Formulas for Simple Ionic Compounds | Breslyn.org
The Criss-Cross Method for Writing Chemical Formulas
How To Write Chemical Equations From Word Descriptions
Empirical and Molecular Formula: How to Determine Empirical Formula from Percentage Composition.
How to write a cosmetic formula
How to create formulas in Microsoft Excel
Writing Ionic Formulas
Excel Formulas and Functions Tutorial
The Simple Songwriting Formula that Changed Everything for Me
How to Make any Chemical Formula under 10 seconds 🔥| Class 10| Prashant Kirad
This Simple Songwriting Formula Will Make You Write Better Songs In 20 Minutes
Writing a General Formula of an Arithmetic Sequence
Common Chemical and Formula list in Chemistry 📝 ||
GCSE Chemistry: How to Write Any Chemical Formula | Master the Skill
Learn how to write the explicit formula given a sequence of numbers
How Chemical Equations are Formed? | Don't Memorise
Formula Writing for Acids: Explanation, Flowchart, and Practice
Writing the Formula for Carbon Dioxide
Formula Making in Chemistry for Class 9 Under 10 Minutes Concept with Ashu sir | Science and fun
Комментарии
 0:10:22
0:10:22
 0:11:44
0:11:44
 0:03:38
0:03:38
 0:05:25
0:05:25
 0:04:17
0:04:17
 0:04:39
0:04:39
 0:05:30
0:05:30
 0:21:56
0:21:56
 0:07:22
0:07:22
 0:20:25
0:20:25
 0:01:38
0:01:38
 0:16:00
0:16:00
 0:12:29
0:12:29
 0:09:45
0:09:45
 0:21:28
0:21:28
 0:18:36
0:18:36
 0:12:53
0:12:53
 0:00:06
0:00:06
 0:06:09
0:06:09
 0:02:20
0:02:20
 0:02:10
0:02:10
 0:07:41
0:07:41
 0:00:34
0:00:34
 0:10:07
0:10:07