filmov
tv
Formula Mass and Molar Mass of a Compound

Показать описание
How to calculate the formula mass and molar mass of a compound. Terms like molecular mass and molecular weight are often used interchangeably with formula mass. The "molecular" term just means you're dealing with a molecular (or covalent) compound.
Thanks for watching! 😀
Follow me on:
#FormulaMass #MolarMass #Chemistry #FormulaWeight #MoleularWeight #PeriodicTable #GeneralChemistry #Mass #Mole #Moles #AtomicMassUnit #amu #AtomicMass #ConversionFactor #Calculations
Thanks for watching! 😀
Follow me on:
#FormulaMass #MolarMass #Chemistry #FormulaWeight #MoleularWeight #PeriodicTable #GeneralChemistry #Mass #Mole #Moles #AtomicMassUnit #amu #AtomicMass #ConversionFactor #Calculations
Formula Mass and Molar Mass of a Compound
How To Calculate The Molar Mass of a Compound - Quick & Easy!
GCSE Chemistry - Relative Formula Mass #24
Difference between Molecular Mass and Formula Mass
How to Calculate Molar Mass (Molecular Weight)
How to Calculate Molar Mass Practice Problems
Formula Mass and Molar Mass of Compounds
Relative Formula Mass - mole concept
Chemistry mcqs #generalknowledgeforallcompetitiveexams #mdcattest #chemistry #shorts #viralreels
How to calculate molecular mass and molecular weight?
What are The Relative Molecular and Formula Mass?
GCSE Chemistry - The Mole (Higher Tier) #25
The mole & Molar Mass - amu - Formula Unit, Mass- Avogadro's Number (with Practice Problem...
Molar Mass | Chemistry
Convert Molar Mass to Moles (2021)
Empirical Formula & Molecular Formula Determination From Percent Composition
Understanding Molecular & Formula Mass in Chemistry
Molar Mass in One Minute
How to calculate molar mass?
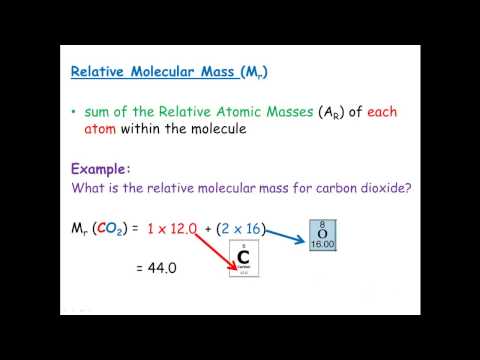
Relative Molecular Mass & Relative Formula Mass
chemistry formula mass NaCl
What is the difference between molecular mass and molar mass?
How to calculate molecular mass/molecular weight
How To Calculate Molar Mass
Комментарии
 0:04:50
0:04:50
 0:11:20
0:11:20
 0:03:59
0:03:59
 0:04:03
0:04:03
 0:03:51
0:03:51
 0:13:11
0:13:11
 0:11:34
0:11:34
 0:05:57
0:05:57
 0:00:56
0:00:56
 0:04:50
0:04:50
 0:03:55
0:03:55
 0:04:29
0:04:29
 0:27:56
0:27:56
 0:07:26
0:07:26
 0:01:25
0:01:25
 0:11:00
0:11:00
 0:34:31
0:34:31
 0:01:00
0:01:00
 0:05:03
0:05:03
 0:01:40
0:01:40
 0:01:08
0:01:08
 0:03:28
0:03:28
 0:04:23
0:04:23
 0:09:00
0:09:00