filmov
tv
18. Introduction to Chemical Equilibrium

Показать описание
MIT 5.111 Principles of Chemical Science, Fall 2014
Instructor: Catherine Drennan
Reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. In this lecture, we discuss the nature of chemical equilibrium and of the chemical equilibrium constant. We start to consider how external factors can “push” the equilibrium in one direction or the other. Physicist and Chemist Nozomi Ando provides an example of why chemical equilibrium is important in living organisms.
License: Creative Commons BY-NC-SA
Instructor: Catherine Drennan
Reactions reach chemical equilibrium when the rate of the forward reaction equals the rate of the reverse reaction. In this lecture, we discuss the nature of chemical equilibrium and of the chemical equilibrium constant. We start to consider how external factors can “push” the equilibrium in one direction or the other. Physicist and Chemist Nozomi Ando provides an example of why chemical equilibrium is important in living organisms.
License: Creative Commons BY-NC-SA
18. Introduction to Chemical Equilibrium
Chemical Equilibria and Reaction Quotients
Equilibrium: Crash Course Chemistry #28
18.1 The Nature of Chemical Equilibrium
Intro to Equilibrium
The Common Ion Effect
Introduction to chemical equilibrium
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
Ionic & Chemical Equilibrium Chemistry IIT JAM / CUET PG 2026: Introduction to Ionic Equilibria!
Introduction to Chemical Equilibrium and the Equilibrium Constant (K) | Chemical Equilibrium
Introduction to Chemical Equilibrium
Introduction to Chemical Equilibrium Concepts (Part 1)
Introduction to Chemical Equilibrium
Introduction to Chemical Equilibrium
Equilibrium Made Easy: How to Solve Chemical Equilibrium Problems
apspc18 ap chemistry introduction to equilibrium
Nexttoppes queens #nexttoppersfeels #opnexttoppersfeels #nexttoppers #nexttopper #prashantbhaiya
Fascinating Chemistry Experiments | Elephant Toothpaste | Amazing Chemistry Experiments #shorts
15.1 Chemical Equilibrium and Equilibrium Constants | General Chemistry
MCQs Chemical Equilibrium || Chemistry Quiz Q-12
🔥Carbon Hybridization Single & Double Bond🔥 #carbonhybridization #iit #jee #chemistry #kotafacto...
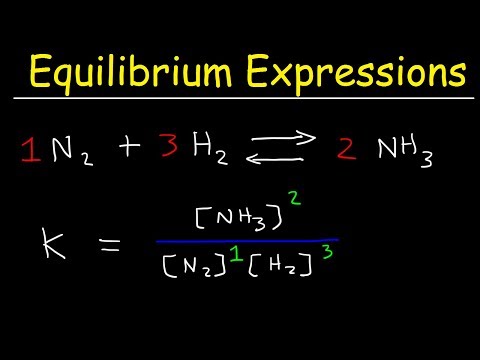
How To Write The Equilibrium Expression For a Chemical Reaction - Law of Mass Action
Why is everyone laughing? 😱😂 #eapcet2025
Redox Reaction #learnwithmansi #class10th #chemicalreaction
Комментарии
 0:47:51
0:47:51
 0:06:48
0:06:48
 0:10:56
0:10:56
 0:10:17
0:10:17
 0:02:33
0:02:33
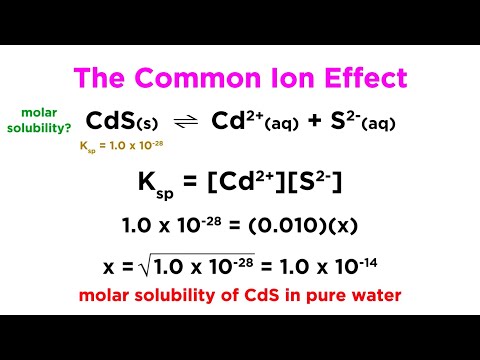 0:04:26
0:04:26
 0:07:17
0:07:17
 0:53:22
0:53:22
 1:10:58
1:10:58
 0:12:09
0:12:09
 0:10:59
0:10:59
 0:08:40
0:08:40
 0:23:47
0:23:47
 0:06:33
0:06:33
 0:12:43
0:12:43
 0:04:57
0:04:57
 0:00:18
0:00:18
 0:00:46
0:00:46
 0:28:41
0:28:41
 0:00:16
0:00:16
 0:00:58
0:00:58
 0:05:24
0:05:24
 0:00:37
0:00:37
 0:00:59
0:00:59