filmov
tv
Collision theory and how it explains chemical reactions rates
Показать описание
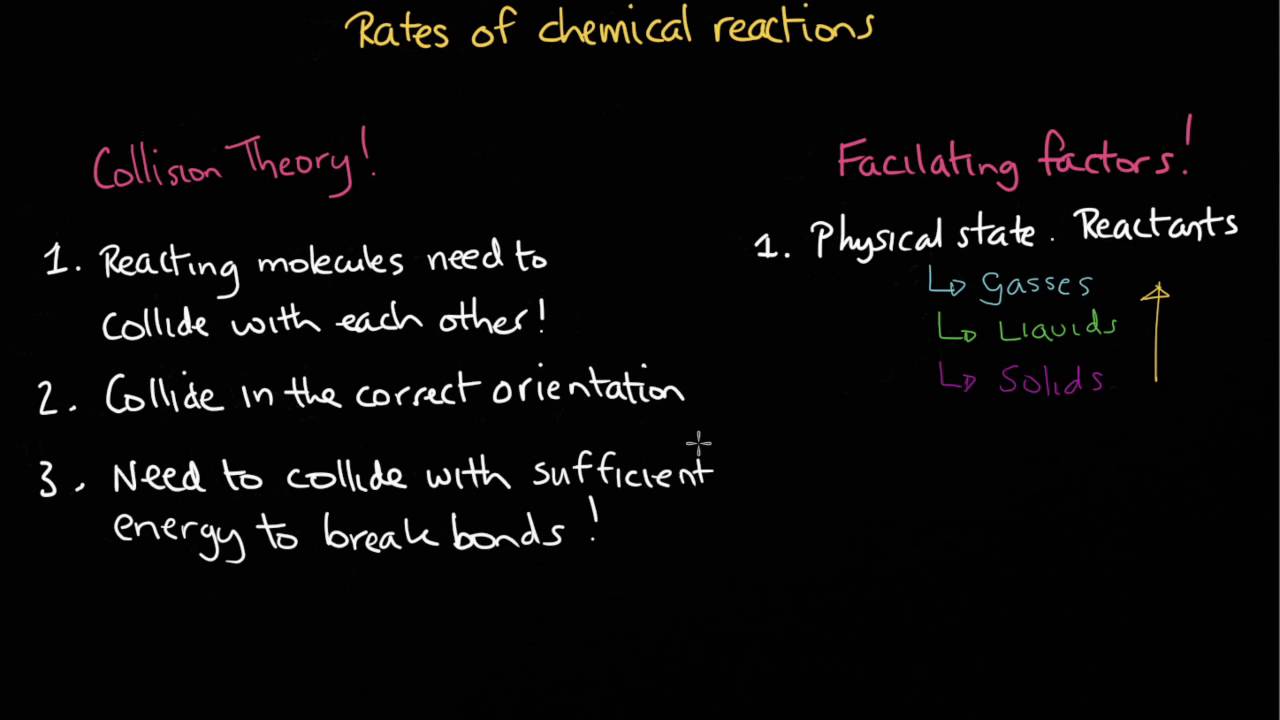
Collision theory states that in order for chemical reactions to occur 1) Reacting molecules need to collide with each other. 2 ) Collide in the correct orientation. 3) Need to collide with sufficient energy to break bonds. This video gives an overview of the facilitating factors that when combined with above allows chemical reactions to occur. For example, how does temperature and concentration affect the rate of reaction? What is the role of a catalyst? How does a catalyst work?
 0:02:13
0:02:13
 0:02:29
0:02:29
 0:08:48
0:08:48
 0:02:59
0:02:59
 0:02:05
0:02:05
 0:05:15
0:05:15
 0:31:50
0:31:50
 0:13:56
0:13:56
 0:03:50
0:03:50
 0:01:53
0:01:53
 0:00:08
0:00:08
 0:16:34
0:16:34
 0:14:57
0:14:57
 0:04:56
0:04:56
 0:02:08
0:02:08
 0:01:36
0:01:36
 0:08:03
0:08:03
 0:02:10
0:02:10
 0:07:28
0:07:28
 0:03:28
0:03:28
 0:04:13
0:04:13
 0:02:36
0:02:36
 0:03:35
0:03:35
 0:05:26
0:05:26