filmov
tv
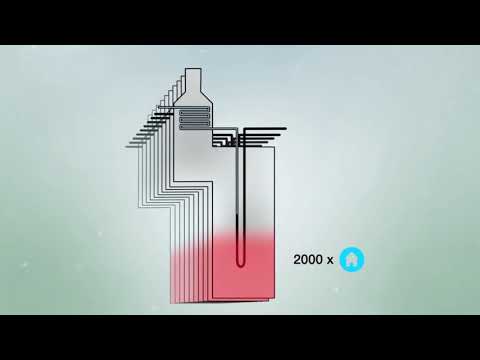
How does ammonia cracking work?

Показать описание
Hydrogen is a critical part of the energy transition as it can provide energy for hard-to-electrify sectors such as long-haul transport, chemicals, iron and steel, refining, marine fuel and power generation, trucking, and power generation. The Hydrogen Council estimates that hydrogen could meet 18% of global energy demand by 2050. #Ammonia is an ideal carrier of hydrogen as it is much easier to compress and transport. When shipped, after arriving at its destination, the ammonia needs to be decomposed, or “cracked,” into hydrogen, before use in the energy value chain. Watch here how the ammonia cracking process works.
How does ammonia cracking work?
Ammonia synthesis How does it work
Hydrogen energy storage in AMMONIA: Fantastic future or fossil fuel scam?
WHAT is Ammonia Cracker Furnace and what can it do for the continuous bright heat treatmnt furnace ?
Cracking Liquid Ammonia
Ammonia—a renewable fuel made from sun, air, and water—could power the globe without carbon
What is ammonia cracking? - Catator
How to transport hydrogen using ammonia (NH3)
Ammonia Cracker - Air-N-Gas Process Technologies
Ammonia cracker production plant | Ammonia cracker gas generator machine | Labh Group
Ammonia cracking at Fraunhofer IMM
What is green ammonia?
Ammonia Cracking for High Purity Hydrogen Production
What is Cracking Dow Chemical Builds New Ethylene Production Plant at Dow Texas Operations
Ammonia-Based Fuels: The Future of Automotive Industry?
Network H2 Webinar 25th February 2022, 'Development of an onboard ammonia cracking system&apos...
Ammonia Cracker Unit
REVOLVE | How To Produce Green Ammonia
Comparative Insights into Low and High Temperature Ammonia Cracking Catalysts #WorldHydrogen2024
Ammonia Cracker Internal structure #mechanicalengineering #machine
Fractory-made Ammonia Decomposition Cracker Furnace in HCS
Commissiong of ammonia cracker heat treatment of high carbon steel
The race to find clean fuel: Ammonia-powered transportation
Ammonia Decomposition/ Cracker Furnace - Electric Heating & Natural Gas Heating
Комментарии
 0:01:28
0:01:28
 0:03:00
0:03:00
 0:12:19
0:12:19
 0:01:29
0:01:29
 0:02:43
0:02:43
 0:03:01
0:03:01
 0:01:55
0:01:55
 0:07:04
0:07:04
 0:01:09
0:01:09
 0:02:36
0:02:36
 0:02:32
0:02:32
 0:01:08
0:01:08
 0:09:53
0:09:53
 0:02:24
0:02:24
 0:05:44
0:05:44
 0:34:25
0:34:25
 0:00:25
0:00:25
 0:01:21
0:01:21
 0:10:57
0:10:57
 0:00:13
0:00:13
 0:01:12
0:01:12
 0:00:15
0:00:15
 0:02:17
0:02:17
 0:02:16
0:02:16