filmov
tv
Calculating Molar Mass from Boiling Point Elevation

Показать описание
This short video shows you how to calculate the molar mass of a nonelectrolyte (van't hoff=1) using boiling point elevation.
Example Question:
5.00 g of an organic solid is dissolved in 100.0 g of benzene. The boiling temperature of this solution is 82.42 °C. The boiling temperature of pure benzene is 80.1 °C; Kb = 2.53 °C /m. What is the molecular weight of the unknown compound?
Example Question:
5.00 g of an organic solid is dissolved in 100.0 g of benzene. The boiling temperature of this solution is 82.42 °C. The boiling temperature of pure benzene is 80.1 °C; Kb = 2.53 °C /m. What is the molecular weight of the unknown compound?
Calculating Molar Mass from Boiling Point Elevation
Week 4 - 4. Calculating molar mass from boiling point elevation
Calculating The Molar Mass of The Unknown Compound From Boiling Point Elevation
How to Calculate Molecular Weight in Boiling Point Elevation | Analytical Chemistry
Calculating the molar mass of a solute using boiling point data (13.104)
Calculating Molar Mass from Freezing Point Depression
Calculating the Molar Mass by Boiling-Point Elevation
Finding the Molar Mass of a Volatile Liquid
CBSE 12 Board Exam 2025 | Chemistry MCQs Series | Solutions Must-Know 100 MCQs
Determining Molar Mass of Unknown using Freezing Point Depression (Colligative Properties)
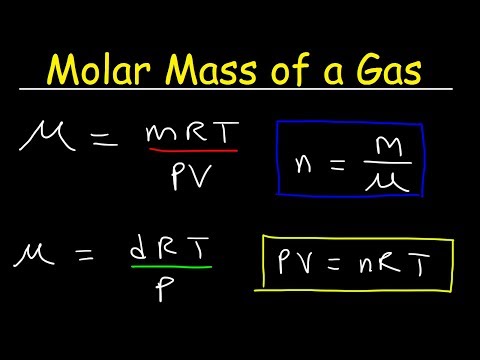
Molar Mass of a Gas at STP - Equations & Formulas, Chemistry Practice Problems
Calculations for Lab 5 Molar Mass Determination by boiling point elevation
General Chemistry II - Freezing Point Depression - Solving for Molar Mass
Determine molecular weight by boiling point elevation. #shortsfeed #viral #viralvideo #chemistry
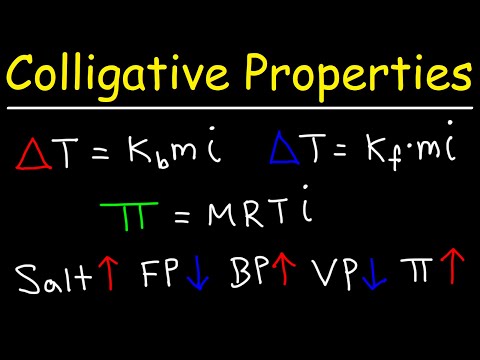
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
Calculating The Boiling Point Of Solution From Boiling Point Elevation
Calculating the Boiling Point of a Solution
Determining Molar Mass with Colligative Properties Notes honors
Calculating the boiling point of a solution
Calculating boiling point elevation
Boiling point elevation of Dilute solutions and Molecular mass determination (Thermodynamics 1)
ALEKS: Using a solution freezing point to calculate molar mass
Trick to Calculate Atomic Mass of elements (Z = 21 - 30 ) #shorts #reels #chemistry #tricks
How to determine the Molecular weight of solute from Boiling Point Elevation | Thermodynamics | Phys
Комментарии
 0:02:30
0:02:30
 0:05:05
0:05:05
 0:06:53
0:06:53
 0:07:51
0:07:51
 0:09:46
0:09:46
 0:02:44
0:02:44
 0:00:19
0:00:19
 0:04:12
0:04:12
 1:26:24
1:26:24
 0:04:41
0:04:41
 0:10:41
0:10:41
 0:05:38
0:05:38
 0:03:37
0:03:37
 0:00:20
0:00:20
 0:25:23
0:25:23
 0:08:12
0:08:12
 0:02:59
0:02:59
 0:09:08
0:09:08
 0:06:31
0:06:31
 0:02:52
0:02:52
 0:22:24
0:22:24
 0:05:35
0:05:35
 0:00:58
0:00:58
 0:01:06
0:01:06