filmov
tv
The Strong Nuclear Force

Показать описание
In the vastness of the universe, the forces of gravity and electromagnetism are familiar aspects of our daily lives. However, the Strong Nuclear Force and Weak Nuclear Force operate on a micro-scale, beyond our perceptible experiences. Among these forces, the Strong Nuclear Force, responsible for holding particles within the atomic nucleus together, stands out as the most powerful.
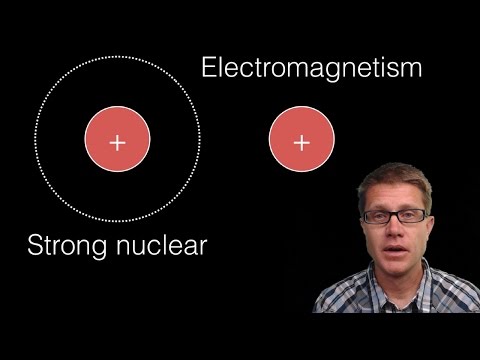
Within the nucleus of an atom, there exist neutrons and protons. While protons carry a positive electric charge, neutrons are neutral particles. Understanding that like charges repel and opposite charges attract, we recognize the electrostatic force between charged particles. Protons, being positively charged, would naturally repel each other within the atomic nucleus. If left unchecked, this repulsive force would cause the nucleus to break apart. However, our surroundings demonstrate that atomic nuclei remain stable. Neutrons play a crucial role in reducing the effects of electrostatic force. By inserting themselves between protons, neutrons increase the distance between them, mitigating the repulsive force.
Yet, neutrons alone are not sufficient to keep the atomic nucleus intact. Here enters the “Strong Nuclear Force,” which binds quarks, the elementary particles constituting nucleons (protons and neutrons), and plays a vital role in preserving the structure of the atomic nucleus.
The Strong Nuclear Force among Quarks:
Quarks, fundamental particles constituting nucleons, possess a mass approximately 1% of a proton’s mass. There are six types of quarks: up, down, charm, strange, top, and bottom. Protons consist of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks. The Strong Nuclear Force, also known as gluons, facilitates the interaction between quarks.
Gluons, acting as carriers of the Strong Nuclear Force, enable the transmission of energy between quarks. The force between quarks is so intense that they cannot be separated. This force not only prevents quarks from dispersing but also hinders their separation. Thus, the structure of protons and neutrons is maintained.
Within the nucleus of an atom, there exist neutrons and protons. While protons carry a positive electric charge, neutrons are neutral particles. Understanding that like charges repel and opposite charges attract, we recognize the electrostatic force between charged particles. Protons, being positively charged, would naturally repel each other within the atomic nucleus. If left unchecked, this repulsive force would cause the nucleus to break apart. However, our surroundings demonstrate that atomic nuclei remain stable. Neutrons play a crucial role in reducing the effects of electrostatic force. By inserting themselves between protons, neutrons increase the distance between them, mitigating the repulsive force.
Yet, neutrons alone are not sufficient to keep the atomic nucleus intact. Here enters the “Strong Nuclear Force,” which binds quarks, the elementary particles constituting nucleons (protons and neutrons), and plays a vital role in preserving the structure of the atomic nucleus.
The Strong Nuclear Force among Quarks:
Quarks, fundamental particles constituting nucleons, possess a mass approximately 1% of a proton’s mass. There are six types of quarks: up, down, charm, strange, top, and bottom. Protons consist of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks. The Strong Nuclear Force, also known as gluons, facilitates the interaction between quarks.
Gluons, acting as carriers of the Strong Nuclear Force, enable the transmission of energy between quarks. The force between quarks is so intense that they cannot be separated. This force not only prevents quarks from dispersing but also hinders their separation. Thus, the structure of protons and neutrons is maintained.
Комментарии
 0:05:06
0:05:06
 0:04:25
0:04:25
 0:11:05
0:11:05
 0:06:38
0:06:38
 0:21:37
0:21:37
 0:01:00
0:01:00
 0:02:51
0:02:51
 0:16:07
0:16:07
 0:06:21
0:06:21
 0:09:37
0:09:37
 0:06:33
0:06:33
 0:02:00
0:02:00
 0:06:23
0:06:23
 0:00:26
0:00:26
 0:06:24
0:06:24
 0:03:32
0:03:32
 0:08:34
0:08:34
 0:03:37
0:03:37
 0:15:59
0:15:59
 0:03:58
0:03:58
 0:02:09
0:02:09
 0:00:31
0:00:31
 0:08:12
0:08:12
 0:00:57
0:00:57