filmov
tv
√ What are Emulsions and How Do They Work?

Показать описание
📢 Receive Comprehensive Mathematics Practice Papers Weekly for FREE 😊
If olive oil is shaken in the water, the oil temporarily breaks up into drops. These oil droplets are not stable as they cannot interact strongly with the water molecules. The droplets rise to the surface and combine to form a separate layer of oil floating on the water.
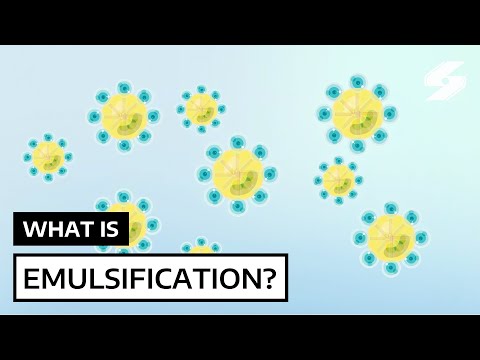
Small oil droplets can be stabilised and dispersed in water by a surfactant. Soap is a surfactant and an emulsifier. The soap is an emulsifier as it causes the oil droplets to remain stable and dispersed throughout the water. The long hydrocarbon tails of the soap dissolve in the oil droplets and the charged heads remain on the surface of the droplets to interact with the water. This change in the surface properties of the oil droplets keeps the emulsion stable.
• A surfactant is a substance that decreases the surface tension of water or another solvent that disperses oil, dirt or grease as small particles throughout the water.
• The emulsion is a dispersion of small droplets of one liquid throughout another liquid.
A surfactant must be added to all emulsions to stabilise them. Mayonnaise, for example, is an oil-in-¬water emulsion that consists of fine droplets of salad oil in vinegar. The emulsifier in mayonnaise is lecithin which is present in the egg yolk. Butter and cheese are examples of emulsions in which aqueous droplets are dispersed throughout the oil phase. Synthetic emulsifiers are used to stabilise emulsions in cosmetics.
0:00 Introduction
0:08 5.5.2 SURFACTANTS industrial chemistry Year 12 Chemistry
0:49 What is an Emulsion?
1:57 Emulsifiers
5:03 Other Emulsions
6:23 Unstable Emulsions
7:21 Question 1
If olive oil is shaken in the water, the oil temporarily breaks up into drops. These oil droplets are not stable as they cannot interact strongly with the water molecules. The droplets rise to the surface and combine to form a separate layer of oil floating on the water.
Small oil droplets can be stabilised and dispersed in water by a surfactant. Soap is a surfactant and an emulsifier. The soap is an emulsifier as it causes the oil droplets to remain stable and dispersed throughout the water. The long hydrocarbon tails of the soap dissolve in the oil droplets and the charged heads remain on the surface of the droplets to interact with the water. This change in the surface properties of the oil droplets keeps the emulsion stable.
• A surfactant is a substance that decreases the surface tension of water or another solvent that disperses oil, dirt or grease as small particles throughout the water.
• The emulsion is a dispersion of small droplets of one liquid throughout another liquid.
A surfactant must be added to all emulsions to stabilise them. Mayonnaise, for example, is an oil-in-¬water emulsion that consists of fine droplets of salad oil in vinegar. The emulsifier in mayonnaise is lecithin which is present in the egg yolk. Butter and cheese are examples of emulsions in which aqueous droplets are dispersed throughout the oil phase. Synthetic emulsifiers are used to stabilise emulsions in cosmetics.
0:00 Introduction
0:08 5.5.2 SURFACTANTS industrial chemistry Year 12 Chemistry
0:49 What is an Emulsion?
1:57 Emulsifiers
5:03 Other Emulsions
6:23 Unstable Emulsions
7:21 Question 1
Комментарии
 0:03:08
0:03:08
 0:11:18
0:11:18
 0:05:43
0:05:43
 0:04:34
0:04:34
 0:04:38
0:04:38
 0:02:56
0:02:56
 0:15:40
0:15:40
 0:03:04
0:03:04
 0:03:13
0:03:13
 0:03:28
0:03:28
 0:03:08
0:03:08
 0:09:49
0:09:49
 0:00:49
0:00:49
 0:11:07
0:11:07
 0:12:35
0:12:35
 0:00:30
0:00:30
 0:03:23
0:03:23
 0:03:36
0:03:36
 0:01:29
0:01:29
 0:05:33
0:05:33
 0:04:58
0:04:58
 0:02:27
0:02:27
 0:04:40
0:04:40
 0:08:20
0:08:20