filmov
tv
Easy Way To Determine Aromaticity: Aromatic, Antiaromatic, Nonaromatic

Показать описание
Quick and easy tricks to determine whether molecules are aromatic, antiaromatic, or nonaromatic... especially helpful for #dat questions. Common confusions involving hetercyclics and charges is cleared up in this video. And you don't have to memorize Hückel's Rule to determine whether molecules are aromatic, antiaromatic, or non aromatic.
#mcat #dat #organicchemistry #testpreparation @PraxisAcademic #organicchemistrytricks #chemistrytricks #dentaladmissionstest #predental #dat #datquestionoftheday #datprep #predentalstudent #datbootcamp #datdestroyer #pat #dattest #datpractice #bohrprep #kaplan #angleranking #dentalexam #patpractice #holepunching #keyhole #cubecounting #topfrontend #patquestionoftheday #weeklydatquiz #dentaladmissiontest #dentalschool #predentistry #datstudying #loveteeth #predentalclub #predentalsociety #questionoftheday
#mcat #dat #organicchemistry #testpreparation @PraxisAcademic #organicchemistrytricks #chemistrytricks #dentaladmissionstest #predental #dat #datquestionoftheday #datprep #predentalstudent #datbootcamp #datdestroyer #pat #dattest #datpractice #bohrprep #kaplan #angleranking #dentalexam #patpractice #holepunching #keyhole #cubecounting #topfrontend #patquestionoftheday #weeklydatquiz #dentaladmissiontest #dentalschool #predentistry #datstudying #loveteeth #predentalclub #predentalsociety #questionoftheday
Aromatic, Antiaromatic, or Nonaromatic - Huckel's Rule - 4n+2 - Heterocycles
Aromaticity and Huckel's Rule
Aromatic Antiaromatic and Nonaromatic Compounds | Super Easy Trick
Easy Way To Determine Aromaticity: Aromatic, Antiaromatic, Nonaromatic
How to Determine Aromaticity in Charged Molecules
What Is An Aromatic Compound?
Huckel’s Rule for Aromaticity + Time-saving Shortcut
💯Aromaticity Trick in Organic chemistry | Class 11 | IIT JEE & NEET | Vineet khatri
PYQ NEET/JEE Series part 44/hydrocarbons chemistry class12/aromaticity organic chemistry/ solutions
How to Identify Aromatic, Anti Aromatic, and Non Aromatic Compound || Super Trick || Organic
Aromaticity in Organic Chemistry | Class 11| IIT JEE & NEET | Vineet Khatri | ATP STAR NEET
Aromaticity Part 1 - Cyclic Planar Conjugated and Huckel's Rule
Aromatic, Antiaromatic, or Nonaromatic Practice Session #1
17.2 Aromatic vs Antiaromatic vs Nonaromatic | Organic Chemistry
Recognizing and Understanding Aromaticity (Worksheet Solutions Walkthrough)
What is Huckel Rule ? | How to Find Types of Aromaticity ? | One Minute Chemistry
3 Criteria for Identifying Aromaticity with Practice Problems!
Organic chemistry - Identifying aromaticity of molecules
How to Determine Aromaticity and Anti-Aromaticity
Huckel rule of aromaticity #ytshorts #chemistry #viral #latestvideo
Aromaticity, Hückel's Rule, and Chemical Equivalence in NMR: Crash Course Organic Chemistry #3...
What is Aromatic? | Rules of Aromaticity
Organic Chemistry || GOC 06 : Aromatic , Anti Aromatic and Non-Aromatic Compounds JEE MAINS/NEET
Trick to Identify Given Compound is Armatic or Anti Aromatic
Комментарии
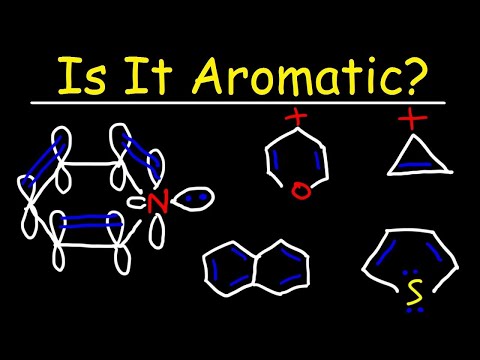 0:10:43
0:10:43
 0:10:00
0:10:00
 0:22:22
0:22:22
 0:10:57
0:10:57
 0:04:26
0:04:26
 0:03:58
0:03:58
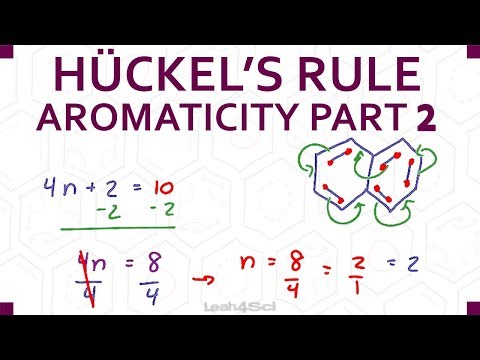 0:11:00
0:11:00
 0:00:51
0:00:51
 0:01:58
0:01:58
 0:11:04
0:11:04
 0:11:34
0:11:34
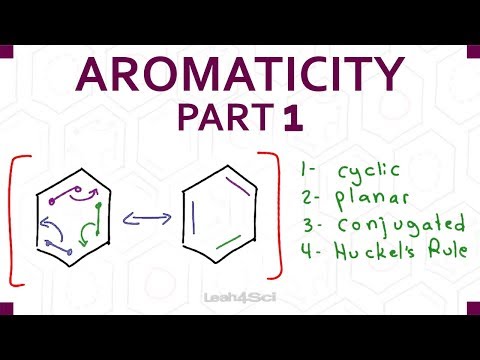 0:10:12
0:10:12
 0:15:35
0:15:35
 0:35:21
0:35:21
 0:13:27
0:13:27
 0:01:46
0:01:46
 0:15:41
0:15:41
 0:06:12
0:06:12
 0:34:11
0:34:11
 0:00:11
0:00:11
 0:13:31
0:13:31
 0:22:12
0:22:12
 0:55:30
0:55:30
 0:05:41
0:05:41