filmov
tv
Equilibrium Practice Problems - The ICE Box Method - AP Chem Unit 7, Topic 7a

Показать описание
In this video, Mr. Krug shows students how to solve equilibrium problems using the ICE (or RICE) box method, using the information you know in a problem to solve for the information you don't know. He works several different types of equilibrium problems and focuses on the most common types of equilibrium problems presented in general chemistry classes.
00:00 Introduction
00:20 The ICE Box Method
01:56 Example Problem No 1
09:32 Example Problem No 2
15:14 Example Problem No 3
20:21 Conclusion
00:00 Introduction
00:20 The ICE Box Method
01:56 Example Problem No 1
09:32 Example Problem No 2
15:14 Example Problem No 3
20:21 Conclusion
General Chemistry II - Equilibrium - Solving for Kc
Statics Example: 2D Rigid Body Equilibrium
ICE Table Practice Problems - Initial Concentration, Equilibrium Concentration, Kc (Part 1)
Equilibrium Made Easy: How to Solve Chemical Equilibrium Problems
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
Practice Problem: Calculating Equilibrium Concentrations
Tricks to Solve Equilibrium Questions easily
Plus One Christmas Exam | Chemistry | Equilibrium | Important 10 Questions | Exam Winner
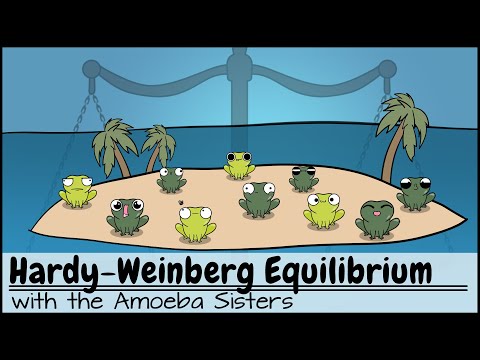
Hardy-Weinberg Equilibrium: Quick Revision in 10 Minutes | NEET 2025 | Class 12 Biology | With TANIA
Worked example: Using Le Chȃtelier’s principle to predict shifts in equilibrium | Khan Academy
Equilibrium Exam Question
ICE Tables: Initial Concentration, Equilibrium Constant Expression - Chemical Equilibrium Problems.
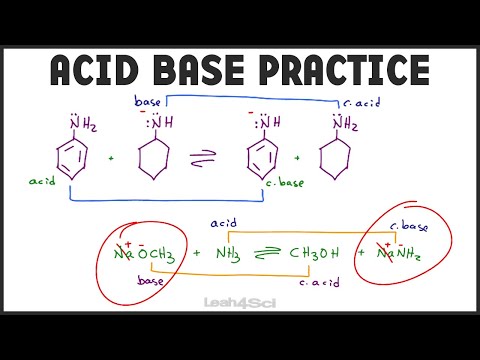
Acid Base Equilibrium Practice - Organic Chemistry
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry
15.3 Equilibrium Calculations Using ICE Charts (aka ICE Tables) | General Chemistry
Nash Equilibrium Examples
The easy way to solve static equilibrium using Sine rule
How to solve Rotational Equilibrium Problems
Hardy-Weinberg Equilibrium
Le Chatelier's Principle
Equilibrium Constant Grade 12: Exam
Chemical equilibrium|Equilibrium constant|Chemistry
Equilibrium Constant Practice Problem
Make sure you completely understand CHEMICAL EQUILIBRIUM + reaction Rates for the TEAS exam
Комментарии
 0:05:17
0:05:17
 0:05:59
0:05:59
 0:09:34
0:09:34
 0:12:43
0:12:43
 0:53:22
0:53:22
 0:03:02
0:03:02
 0:12:00
0:12:00
 0:18:50
0:18:50
 0:08:43
0:08:43
 0:03:58
0:03:58
 0:06:49
0:06:49
 0:29:02
0:29:02
 0:11:07
0:11:07
 0:06:20
0:06:20
 0:23:29
0:23:29
 0:05:14
0:05:14
 0:00:16
0:00:16
 0:17:09
0:17:09
 0:09:36
0:09:36
 0:26:40
0:26:40
 0:06:48
0:06:48
 0:00:06
0:00:06
 0:05:16
0:05:16
 0:00:54
0:00:54