filmov
tv
Chemical Thermodynamics 3.12 - Heat Capacity
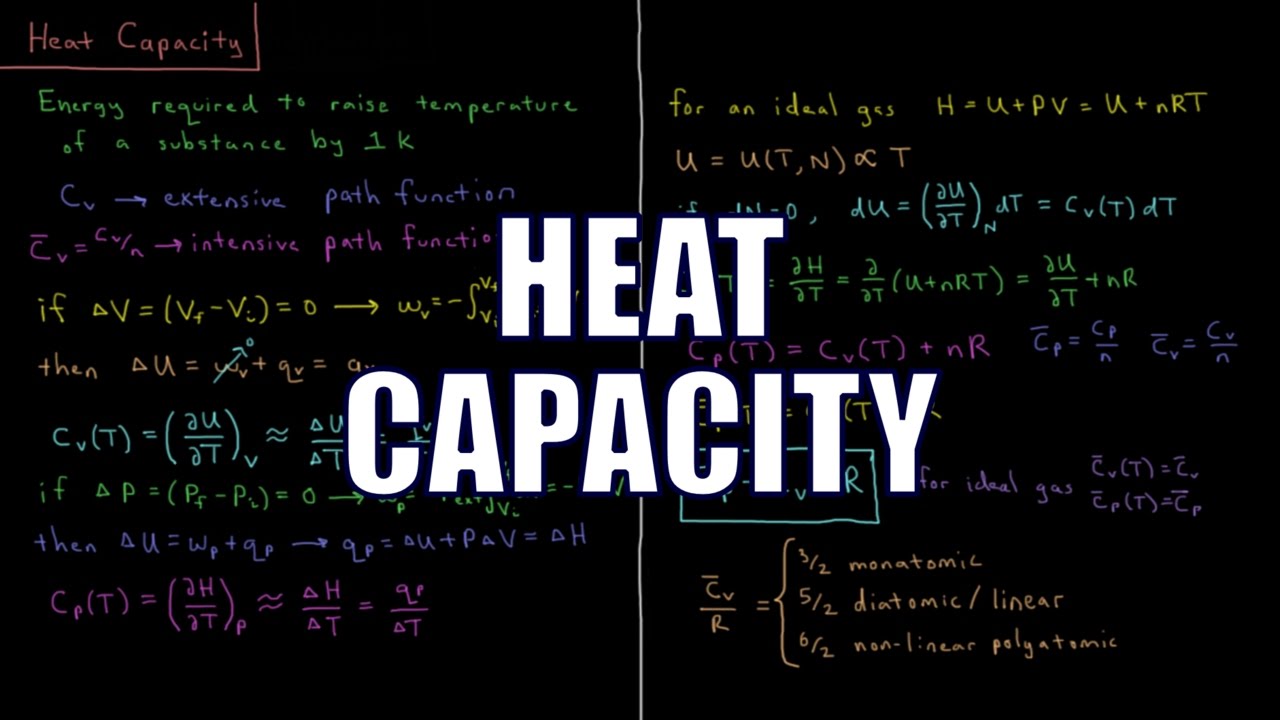
Показать описание
Short physical chemistry lecture on the constant pressure and constant volume heat capacities of ideal gases.
The constant volume heat capacity of a system is defined as the partial derivative of the internal energy with respect to temperature. The constant pressure heat capacity of a system is defined as the partial derivative of the enthlapy with respect to temperature.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
The constant volume heat capacity of a system is defined as the partial derivative of the internal energy with respect to temperature. The constant pressure heat capacity of a system is defined as the partial derivative of the enthlapy with respect to temperature.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Enthalpy | Thermodynamics
Thermodynamics 03 || Heat : Specific heat Capacities Of Gases : Cp and Cv JEE MAINS/NEET||
One shot | 4 chemical thermodynamics chemistry class 12 MAHARASHTRA BOARD with handwritten notes
4. Chemical Thermodynamics L- 3 Class12th || Target Batch 2024 || By :- Abhishek Sir Chemistry ASC
Enthalpy - Chemical Thermodynamics - Chemistry Class 12
Hess's Law Problems & Enthalpy Change - Chemistry
CBSE BOARD- CHEMISTRY |CLASS 11TH - THERMODYNAMICS - heat of Reaction | Lec - 09 |A K EDUCATION
Thermodynamics: Crash Course Physics #23
part-3 ch-4 chemical thermodynamics class 12 science maharashtra board new syllabus Chemistry NIE
The First Law of Thermodynamics: Internal Energy, Heat, and Work
Thermodynamics In Just 30 Minutes! | REVISION - Super Quick! JEE & NEET Chemistry | Pahul Sir
Heat of Reaction Numerical 1 - Chemical Thermodynamics - Chemistry Class 12
Basic Concepts of Thermodynamics (Animation)
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
Lecture -3 ch-4 chemical thermodynamics Chemistry Class 12th #Conceptbatch New Indian era #nie
Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes
Enthalpy of Formation Problems 3 - Chemical Thermodynamics - Chemistry Class 12
Chemical Thermodynamics Class 12th Chemistry Part 2
Chemical Thermodynamics 3.12 - Heat Capacity (Old Version)
Enthalpy: Crash Course Chemistry #18
Change of Enthalpy Numerical 3 - Chemical Thermodynamics - Chemistry Class 12
Class 11th Chemistry - Thermodynamics | Thermodynamics Class 11 Chemistry by GlobalShiksha.com
Комментарии
 0:11:27
0:11:27
 0:08:12
0:08:12
 0:10:55
0:10:55
 0:34:56
0:34:56
 0:57:31
0:57:31
 0:52:45
0:52:45
 0:05:25
0:05:25
 0:14:03
0:14:03
 0:41:50
0:41:50
 0:10:04
0:10:04
 0:49:23
0:49:23
 0:05:44
0:05:44
 0:31:05
0:31:05
 0:03:54
0:03:54
 0:10:57
0:10:57
 0:01:53
0:01:53
 0:59:37
0:59:37
 0:04:11
0:04:11
 0:08:30
0:08:30
 1:06:40
1:06:40
 0:08:12
0:08:12
 0:11:24
0:11:24
 0:03:48
0:03:48
 0:23:36
0:23:36