filmov
tv
Does Sodium (Na) Form a Cation or Anion?

Показать описание
In chemistry it is important to understand the key concept of ions and the terminology. There are two main types of ions, cations and anions. In this video we'll look at whether Sodium (Na) forms a cation or an anion.
When you see an element or a compound with a + or minus sign after it, that tells you that it is an ion.
Cations have positive signs after them. You can remember this because the “t” in Cation ion as a + sign. Anions have a negative sign. Think of it as A Negative Ion (ANion).
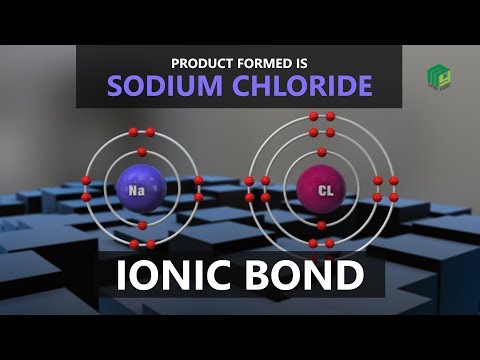
When we think about chemical bonding with ionic compounds, we always have pairs. For example, common table salt, NaCl. There is a big difference in the electronegativity bt Na and Cl which makes it an ionic compound – made up of ions.
Cl is more electronegative than Na and pulls a valence electron away from Na.
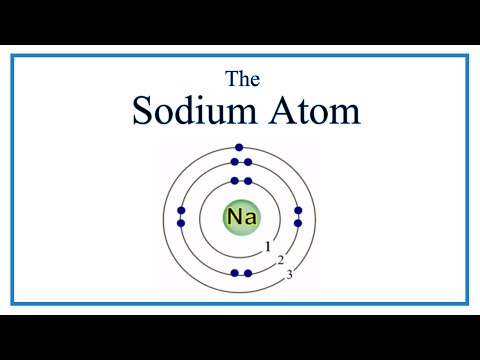
Na has now lost an electron. Since electrons are negative, Na has lost a negative charge. It becomes positive, called a cation. This is the Sodium cation, Na+ .
Cl has gained a negative charge (an electron) and it becomes negative. Called an anion. It is called the Chloride anion, Cl- .
The oppositely charged atoms attract and you have an ionic bond.
We also have what are called polyatomic ions. These are made up of several atoms bonded together. The two main polyatomic cations are NH4 + and H3O + . The other major polyatomic ions are anions.
Resources:
When you see an element or a compound with a + or minus sign after it, that tells you that it is an ion.
Cations have positive signs after them. You can remember this because the “t” in Cation ion as a + sign. Anions have a negative sign. Think of it as A Negative Ion (ANion).
When we think about chemical bonding with ionic compounds, we always have pairs. For example, common table salt, NaCl. There is a big difference in the electronegativity bt Na and Cl which makes it an ionic compound – made up of ions.
Cl is more electronegative than Na and pulls a valence electron away from Na.
Na has now lost an electron. Since electrons are negative, Na has lost a negative charge. It becomes positive, called a cation. This is the Sodium cation, Na+ .
Cl has gained a negative charge (an electron) and it becomes negative. Called an anion. It is called the Chloride anion, Cl- .
The oppositely charged atoms attract and you have an ionic bond.
We also have what are called polyatomic ions. These are made up of several atoms bonded together. The two main polyatomic cations are NH4 + and H3O + . The other major polyatomic ions are anions.
Resources:
Комментарии
 0:01:41
0:01:41
 0:02:14
0:02:14
 0:02:47
0:02:47
 0:02:46
0:02:46
 0:01:31
0:01:31
 0:01:01
0:01:01
 0:00:50
0:00:50
 0:00:28
0:00:28
 0:03:38
0:03:38
 0:00:23
0:00:23
 0:05:51
0:05:51
 0:00:18
0:00:18
 0:01:47
0:01:47
 0:01:51
0:01:51
 0:00:57
0:00:57
 0:04:43
0:04:43
 0:02:46
0:02:46
 0:06:25
0:06:25
 0:00:25
0:00:25
 0:00:46
0:00:46
 0:00:32
0:00:32
 0:02:23
0:02:23
 0:20:45
0:20:45
 0:00:58
0:00:58