filmov
tv
How To Reduce Oxidation in New England IPA - Brew Dudes

Показать описание
Oxidation in New England IPA is a real problem. This week, we discuss ways to reduce it.
We have been getting several questions about oxidation in NEIPA lately. We through in this video we would discuss observations and techniques we have used to be pretty successful in preventing our NEIPAs (or any beer) from turning a murky brown/purple mess with reduced hop aromatics.
Our gold standard is to transfer from fermentor to keg under a closed transfer system. What this means is that you move beer from one CO2 filled container (the fermentor) to another completely CO2 filled container (the keg).
We discuss the challenges faces with bottle conditioning as well as some of the chemical solutions to the problem that we've read and seen floating in various beer interweb places!
Tell us about your practices for limiting oxidation in your NEIPAs!
Cheers!
Check out our blog:
#neipa #oxidation #beer
We have been getting several questions about oxidation in NEIPA lately. We through in this video we would discuss observations and techniques we have used to be pretty successful in preventing our NEIPAs (or any beer) from turning a murky brown/purple mess with reduced hop aromatics.
Our gold standard is to transfer from fermentor to keg under a closed transfer system. What this means is that you move beer from one CO2 filled container (the fermentor) to another completely CO2 filled container (the keg).
We discuss the challenges faces with bottle conditioning as well as some of the chemical solutions to the problem that we've read and seen floating in various beer interweb places!
Tell us about your practices for limiting oxidation in your NEIPAs!
Cheers!
Check out our blog:
#neipa #oxidation #beer
Oxidation-Reduction Reactions
Oxidation and Reduction Reactions - Basic Introduction
Reduction vs.Oxidation in Southwest Pottery - Q and A
How To Reduce Oxidation in New England IPA - Brew Dudes
Introduction to Oxidation Reduction (Redox) Reactions
GCSE Chemistry - Oxidation and Reduction - Redox Reactions #39 (Higher Tier)
Oxidation and Reduction Reactions
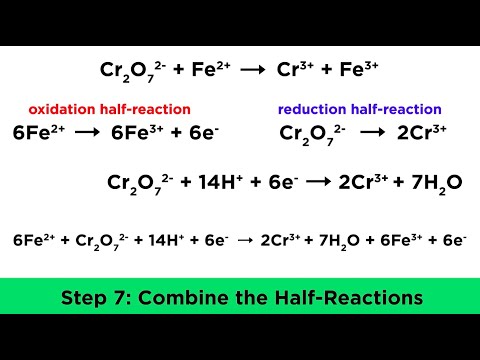
Oxidation and Reduction (Redox) Reactions Step-by-Step Example
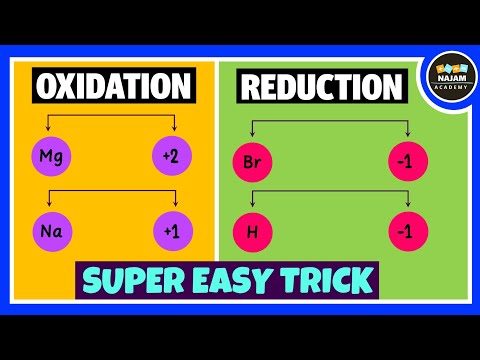
Oxidizing and Reducing Agents | Easy Trick
What Is Oxidation – Dr.Berg on Free Radicals and Antioxidants
The Oxidation Reduction Question that Tricks Everyone!
Oxidation and Reduction
How To Calculate Oxidation Numbers - Basic Introduction
What is Oxidation Reduction & Redox reaction || Diffrence bw Oxidation reaction, Reduction react...
Oxidation vs. Reduction, What are Oxidation and Reduction Reactions in Everyday Life?
Intro to Oxidation and Reduction Reactions in Organic Chemistry
Oxidation and reduction | Redox reactions and electrochemistry | Chemistry | Khan Academy
How to Reduce Cholesterol Oxidation
Balancing Redox Reactions in Acidic and Basic Conditions
Oxidation and Reduction Explained in 2023 Jamb Chemistry Examination
Redox Reactions: Crash Course Chemistry #10
How Oxidation leads to Inflammation and Plaque
Reduction of CO2 and Oxidation of water in photosynthesis.
Oxidation and reduction reaction|| Redox reaction|| class 10|| chemical reaction and equation
Комментарии
 0:03:52
0:03:52
 0:16:05
0:16:05
 0:04:01
0:04:01
 0:15:10
0:15:10
 0:13:05
0:13:05
 0:04:54
0:04:54
 0:12:22
0:12:22
 0:03:56
0:03:56
 0:08:42
0:08:42
 0:06:30
0:06:30
 0:06:19
0:06:19
 0:07:17
0:07:17
 0:31:15
0:31:15
 0:07:04
0:07:04
 0:05:23
0:05:23
 0:13:55
0:13:55
 0:11:04
0:11:04
 0:06:49
0:06:49
 0:07:31
0:07:31
 0:04:35
0:04:35
 0:11:13
0:11:13
 0:03:24
0:03:24
 0:00:31
0:00:31
 0:03:31
0:03:31