filmov
tv
Osmotic Pressure (Example)

Показать описание
The osmotic pressure is surprisingly large: even a relatively dilute solution generates osmotic pressures of several atmospheres.
The solvent can be purified from a solution by applying pressures greater than the osmotic pressure, in a process called reverse osmosis.
The solvent can be purified from a solution by applying pressures greater than the osmotic pressure, in a process called reverse osmosis.
Osmotic Pressure Example
Osmotic Pressure | Example #1
Osmotic Pressure
Osmotic Pressure Example Question | Solutions | Chemistry
Osmosis Animation and Experiments
Osmotic pressure and example - Chemical Engineering Thermodynamics
Osmotic Pressure Example
Osmotic Pressure Example Problem
SP25 - Chem 1220 MT1 Review with DrN
Osmotic Pressure (Example)
Osmosis and Osmotic Pressure || 3D Animated Explanation || class 12th chemistry || Solutions ||
Osmotic Pressure
Osmotic Pressure
Osmosis and Water Potential (Updated)
Hydrostatic and osmotic pressure | Introduction to #edema
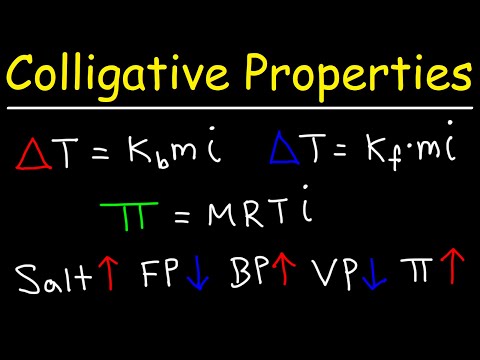
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
AP Chem osmotic pressure + 1 Raoult's Law example
Diffusion, Osmosis and Dialysis (IQOG-CSIC)
Osmotic pressure model: Example (DRAFT video)
Osmosis of Water & Osmotic Pressure | Diffusion of Water
Testing Osmotic Pressure Using Cherries
Chapter 12. Colligative Properties - Osmotic Pressure
Osmosis examples
Osmotic Pressure example - Gen Chem 2
Комментарии
 0:03:04
0:03:04
 0:05:52
0:05:52
 0:03:08
0:03:08
 0:05:10
0:05:10
 0:04:14
0:04:14
 0:12:14
0:12:14
 0:06:32
0:06:32
 0:03:25
0:03:25
 1:28:51
1:28:51
 0:08:11
0:08:11
 0:03:12
0:03:12
 0:02:47
0:02:47
 0:18:50
0:18:50
 0:09:50
0:09:50
 0:06:22
0:06:22
 0:25:23
0:25:23
 0:05:28
0:05:28
 0:01:36
0:01:36
 0:07:34
0:07:34
 0:07:10
0:07:10
 0:01:31
0:01:31
 0:05:54
0:05:54
 0:13:38
0:13:38
 0:03:13
0:03:13