filmov
tv
Mechanism of Nuclear Transport | RAN GTPase Cycle
Показать описание
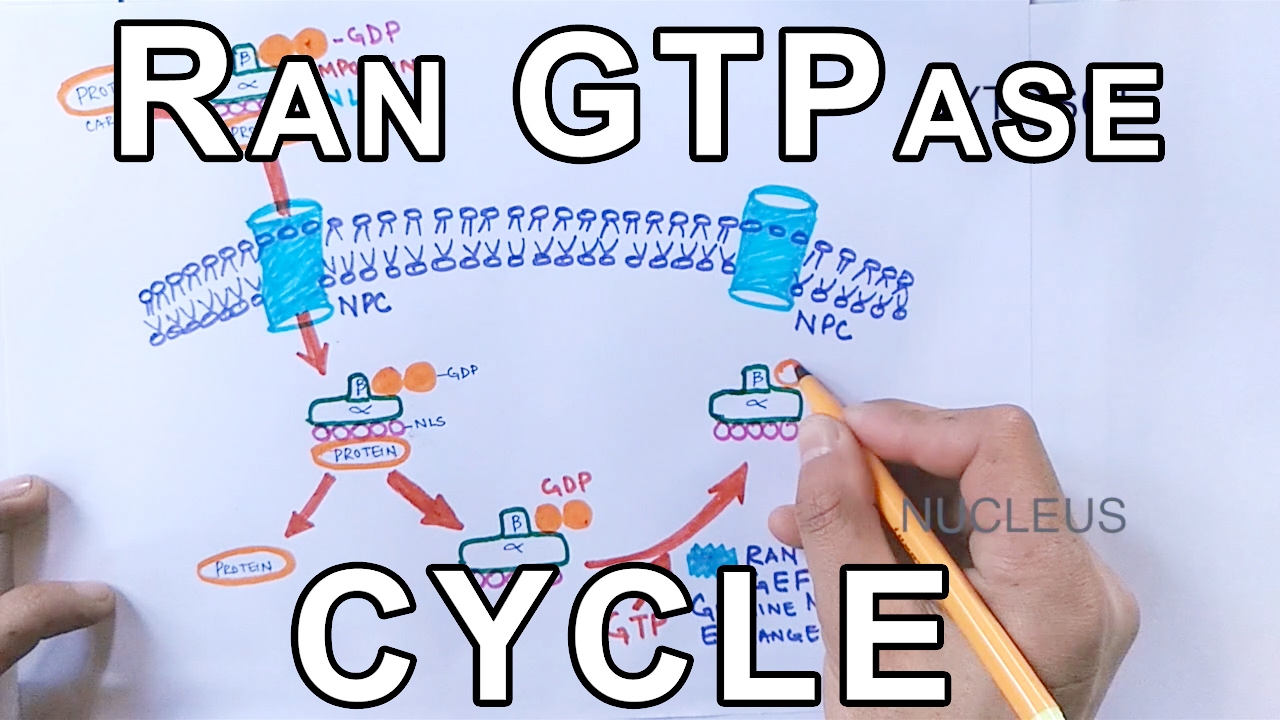
The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecules can enter the nucleus without regulation,[1] macromolecules such as RNA and proteins require association with transport factors like karyopherins called importins to enter the nucleus and exportins to exit.
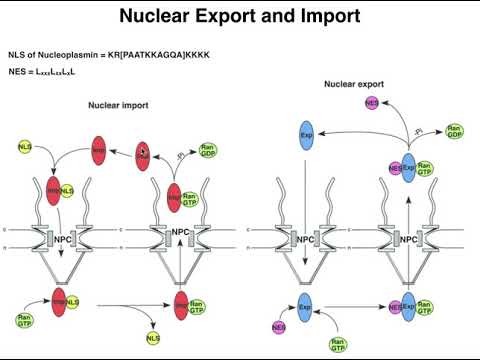
Protein that must be imported to the nucleus from the cytoplasm carry nuclear localization signals (NLS) that are bound by importins. A NLS is a sequence of amino acids that acts as a tag. They are diverse in their composition and most commonly hydrophilic, although hydrophobic sequences have also been documented.[1] Proteins, transfer RNA, and assembled ribosomal subunits are exported from the nucleus due to association with exportins, which bind signaling sequences called nuclear export signals (NES). The ability of both importins and exportins to transport their cargo is regulated by the small Ras related GTPase, Ran.
GTPases are enzymes that bind to a molecule called guanosine triphosphate (GTP) which they then hydrolyze to create guanosine diphosphate (GDP) and release energy. Ran is in a different conformation depending on whether it is bound to GTP or GDP. In its GDP bound state, Ran is capable of binding karyopherins (importins and exportins). Importins release cargo upon binding to RanGTP, while exportins must bind RanGTP to form a ternary complex with their export cargo. The dominant nucleotide binding state of Ran depends on whether it is located in the nucleus (RanGTP) or the cytoplasm (RanGDP).
Protein that must be imported to the nucleus from the cytoplasm carry nuclear localization signals (NLS) that are bound by importins. A NLS is a sequence of amino acids that acts as a tag. They are diverse in their composition and most commonly hydrophilic, although hydrophobic sequences have also been documented.[1] Proteins, transfer RNA, and assembled ribosomal subunits are exported from the nucleus due to association with exportins, which bind signaling sequences called nuclear export signals (NES). The ability of both importins and exportins to transport their cargo is regulated by the small Ras related GTPase, Ran.
GTPases are enzymes that bind to a molecule called guanosine triphosphate (GTP) which they then hydrolyze to create guanosine diphosphate (GDP) and release energy. Ran is in a different conformation depending on whether it is bound to GTP or GDP. In its GDP bound state, Ran is capable of binding karyopherins (importins and exportins). Importins release cargo upon binding to RanGTP, while exportins must bind RanGTP to form a ternary complex with their export cargo. The dominant nucleotide binding state of Ran depends on whether it is located in the nucleus (RanGTP) or the cytoplasm (RanGDP).
Комментарии
 0:04:15
0:04:15
 0:04:30
0:04:30
 0:07:55
0:07:55
 0:09:33
0:09:33
 0:04:32
0:04:32
 0:02:33
0:02:33
 0:16:14
0:16:14
 0:01:20
0:01:20
 0:06:42
0:06:42
 0:14:26
0:14:26
 0:04:22
0:04:22
 0:11:57
0:11:57
 0:08:03
0:08:03
 0:04:02
0:04:02
 0:06:35
0:06:35
 0:22:52
0:22:52
 0:11:40
0:11:40
 0:03:52
0:03:52
 0:09:53
0:09:53
 0:17:05
0:17:05
 0:03:11
0:03:11
 0:10:31
0:10:31
 0:04:13
0:04:13
 0:00:15
0:00:15