filmov
tv
How Did the First Molecules FORM in the Universe? The Start to Life!
Показать описание
CHAPTERS:
0:00 How did the first atoms form?
1:43 Why do atoms form molecules?
2:54 This molecule was the first to form
4:09 Arvin's Transparent head (Keeps Ad)
5:26 Why helium formed BEFORE Hydrogen
7:04 How did water form in the universe?
10:03 How did ORGANIC molecules form?
REFERENCES:
SUMMARY:
To make most complex structures in the universe like trees, and us, you need more than atoms. You need molecules. How do you go from atoms formed in the core of stars, to molecules that can lead to living things? How did the first molecules form?
What is a molecule? It's when two or more atoms connect via a chemical bond. Molecules form when it is more energetically favorable for two or more atoms to form bonds than to remain independent. In other words, the total energy of a system of atoms can be lower than individual atoms on their own. If we wrote the wave functions for hydrogen atoms, we would see that the total energy of the system becomes less when they form a chemical bond.
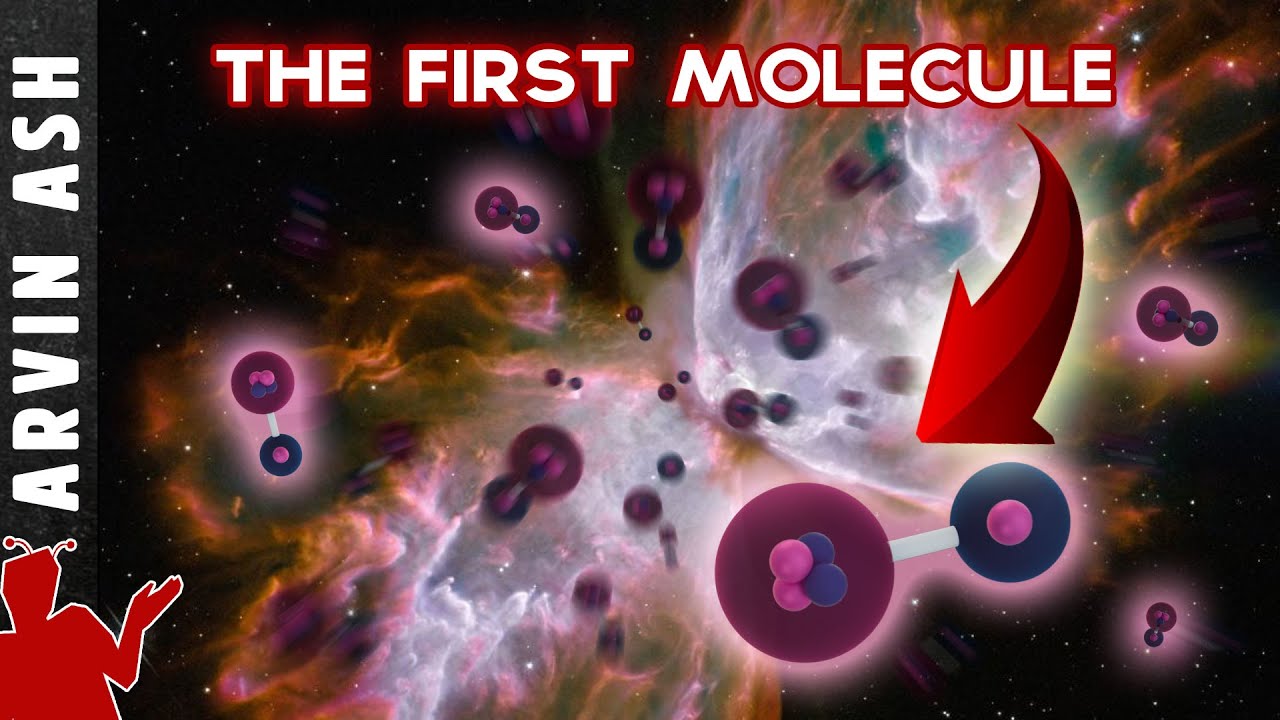
Hydrogen molecules would have formed when the universe was only around 380,000 years old, and are still around today, making them among the oldest molecules in existence. But these were NOT have been the first molecules to form. There is a molecule that formed even earlier than the hydrogen molecule, Helium Hydride, which formed when the universe younger, about 120,000 years old.
This is a positively charged ion consisting of a helium atom bound to a proton, or a hydrogen nucleus. These would have formed first because helium can absorb electrons at higher temperatures than hydrogen. Why? Because it is a noble element and has a higher ionization energy. It takes more energy to remove an electron from helium, so it tends to attract electrons at higher energy levels, so it formed when the universe was hotter at only 120,000 years old, whereas Hydrogen molecules didn’t form until the universe was cooler at 380,000 years old. But helium nuclei formed in very small quantities compared to hydrogen nuclei, just a few percent, so that’s why we don’t have a universe full of mostly helium atoms today.
But how did the molecules necessary for life form? Scientist aren’t completely sure of exactly how, but the evidence appears to point to a plausible scenario:
Over millions of years, helium and hydrogen floating in space condensed into dense clouds. which eventually coalesced into the first stars after about 100 million years. And then these stars burst into life and lit up the cosmos. These early stars, via fusion processes, began producing heavier elements inside their cores. And it was here that the first nuclei of atoms essential for life, like oxygen, carbon, and nitrogen, were formed.
But while stellar processes do make heavier atoms, they don’t produce molecules. As best as we can tell, this is how the first molecules necessary for life appear to have been formed: The first stars came to the end of their life, and exploded as supernovae. This released all the heavier atoms which had formed within their cores into the coldness of space. The atoms cooled to a low enough temperature such that chemical bonds could form between them. These initial reaction would have produced some of the essential molecules for life, like carbon dioxide, methane, hydroxyl and water.
#firstmolecules
These were the precursors for more complex organic molecules, which could have formed in the earth's atmosphere around 4 billion years ago. There is scientific evidence supporting this idea.
There is also the possibility that the first organic molecules came from outside Earth, and not via terrestrial chemical processes. The most recent observational evidence shows that organic compounds seem to be ubiquitous in space: they are found in diffuse clouds, around evolved stars, in dense star-forming nebulae, in proto-planetary disks, in comets, in meteorites and interplanetary dust particles. This evidence seems to indicate that organic molecules may not be all that special in the universe. They may be everywhere. But this picture is not complete because we don’t yet fully understand these interstellar organic molecular formation processes.
Комментарии
 0:12:46
0:12:46
 0:04:23
0:04:23
 0:13:25
0:13:25
 0:00:38
0:00:38
 0:03:10
0:03:10
 0:01:00
0:01:00
 0:10:47
0:10:47
 0:05:05
0:05:05
 0:57:51
0:57:51
 0:00:44
0:00:44
 0:06:45
0:06:45
 0:26:07
0:26:07
 0:05:59
0:05:59
 0:00:35
0:00:35
 0:56:12
0:56:12
 0:01:46
0:01:46
 0:10:05
0:10:05
 0:00:58
0:00:58
 0:01:53
0:01:53
 0:06:08
0:06:08
 0:06:28
0:06:28
 0:04:34
0:04:34
 0:00:06
0:00:06
 0:04:32
0:04:32