filmov
tv
14.4 1st Order Half Life Example Problem

Показать описание
The content of this video is designed to accompany the 12th edition of "Chemistry The Central Science" by Brown, Lemay, Bursten, Murphy, and Woodward. The title of the video corresponds to the section number and topic from the textbook.
©2012 Matthew W. Stoltzfus. All Rights Reserved.
©2012 Matthew W. Stoltzfus. All Rights Reserved.
14.4 1st Order Half Life Example Problem
14.4.3 1st Order Half Life Example Problem 4;19
Chapter 14 Video 7 First Order Half Life Derivation and 1st Order Problem
14.4 Half Life for 1st Order Reactions
Half Life and Rate Constant of 1st Order Reaction [Kinetics]
Half life derivation of first order reaction | first order reaction half life | chemical Kinetics
CH 14 Question about the first order rate decomposition and half life
Half Life of 1st Order Reactions
12.37 | What is the half-life for the first-order decay of carbon-14? (6 C → 7 N + e- ) The rate
For the first order reaction, half life is \( 14 \mathrm{sec} \). T...
For a first order reaction half life is 14 sec. The time required for the initial concentration
For the first order reaction A---B the half life is 3 minutes the time taken for 75% completion of..
Calculation of half life for 1st order reaction #halflife #chemicalkinetics #azhansir #mathechemist
Kinetics: Initial Rates and Integrated Rate Laws
19.5: First Order Half Life
Half life | Radioactivity | Physics | FuseSchool
Chapter 14, Section 4 Half-life
Calculate the half-life of a first order reaction from their rate constants given below
Integrated Rate Laws and Half Life Formula - Nth Order Reaction - Chemical Kinetics
Chemical Kinetics, Chapter 14 – Part 4: Half Lives
Half Life Formula & Example
Half life of Carbon-14 | Today's note | Notes | 15/07/23 | BiologyMadam
5 / 5 - Lecture 15 - Half Lives for 1st order reaction - Using the integrated rate equation
14.5 Integrated Rate Laws and Half Lives
Комментарии
 0:04:20
0:04:20
 0:04:20
0:04:20
 0:11:25
0:11:25
 0:02:49
0:02:49
 0:03:31
0:03:31
 0:03:02
0:03:02
 0:24:56
0:24:56
 0:04:20
0:04:20
 0:06:18
0:06:18
 0:05:23
0:05:23
 0:02:05
0:02:05
 0:03:56
0:03:56
 0:00:50
0:00:50
 0:09:10
0:09:10
 0:07:03
0:07:03
 0:04:54
0:04:54
 0:10:04
0:10:04
 0:01:40
0:01:40
 0:10:02
0:10:02
 0:11:57
0:11:57
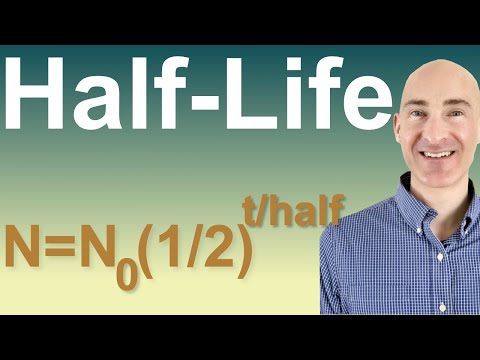 0:02:45
0:02:45
 0:00:22
0:00:22
 0:06:55
0:06:55
 0:15:34
0:15:34