filmov
tv
Neutralization Reaction

Показать описание
030 - Neutralization Reaction
In a neutralization reaction (or acid-base reaction) a proton is transferred from the Brønsted--Lowry acid to the Brønsted--Lowry base. Water is amphoteric and so it can serve as either an acid or a base in a neutralization reaction. The strength of an acid or a base is determined by the ability to donate or receive a proton.
Do you speak another language? Help me translate my videos:
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
In a neutralization reaction (or acid-base reaction) a proton is transferred from the Brønsted--Lowry acid to the Brønsted--Lowry base. Water is amphoteric and so it can serve as either an acid or a base in a neutralization reaction. The strength of an acid or a base is determined by the ability to donate or receive a proton.
Do you speak another language? Help me translate my videos:
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
GCSE Chemistry - Neutralisation Reactions #36
Neutralization Reactions
Acid Base Neutralization Reactions & Net Ionic Equations - Chemistry
A neutralisation reaction C0105
Neutralisation | Acid Bases and Salts | Don't Memorise
Neutralization Reaction
Neutralization Reaction - How to treat a bee sting? | #aumsum #kids #science #education #children
Neutralization Reactions Explained
Class 10 CBSE Science | Rapid Revision in 10 minutes |Acids, Bases, and Salts
Neutralization Reaction Of Acids and Bases | iKen | iKen App | Iken Edu
Neutralization reaction #shorts #science #ytshorts #diy
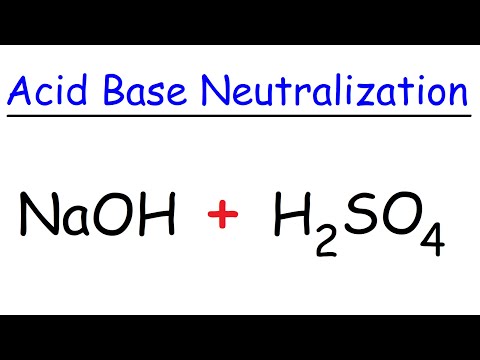
Sodium Hydroxide + Sulfuric Acid - Acid Base Neutralization Reaction
Acid base neutralisation reaction | Chemistry | Khan Academy
Acid Base Neutralization Reactions | Chemistry
Neutralisation
Acid-Base Neutralization Reactions: An Introduction for Students. Worksheet included!
acid-base reaction (HCl + NaOH)
acid base neutralisation reaction experiment video #shorts #scienceexperiment
Acid Base Neutralization Reactions
Neutralization reactions
Neutralization reaction #shorts #science #ytshorts
Neutralization reaction | Acid and Base reaction
Writing and Balancing Neutralization Reactions
GCSE Chemistry - Acids and Bases #34
Комментарии
 0:04:35
0:04:35
 0:06:25
0:06:25
 0:13:33
0:13:33
 0:00:25
0:00:25
 0:02:42
0:02:42
 0:06:03
0:06:03
 0:03:36
0:03:36
 0:10:51
0:10:51
 0:09:44
0:09:44
 0:07:00
0:07:00
 0:00:41
0:00:41
 0:05:32
0:05:32
 0:07:41
0:07:41
 0:08:21
0:08:21
 0:08:19
0:08:19
 0:06:02
0:06:02
 0:00:56
0:00:56
 0:00:26
0:00:26
 0:08:19
0:08:19
 0:09:34
0:09:34
 0:00:28
0:00:28
 0:02:44
0:02:44
 0:04:23
0:04:23
 0:04:37
0:04:37