filmov
tv
Binding Energy per Nucleon

Показать описание
Binding Energy per Nucleon
Nuclear Binding Energy Per Nucleon & Mass Defect Problems - Nuclear Chemistry
Binding Energy per Nucleon and Stability - IB Physics
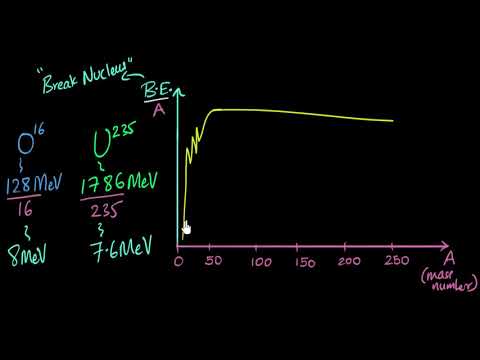
Binding energy graph | Nuclei | Physics | Khan Academy
What is Nuclear Binding Energy? (and BE per nucleon curve)
Binding Energy per Nucleon
A 'cheatsheet' on Binding Energy in nuclear physics
Variation of Binding Energy Per Nucleon Versus Mass Number
Mass deffect and binding energy class 12 | 12th class physics | kpk board, balochistan, punjab board
Calculation of the nuclear binding energy and mass defect
binding energy per nucleon | B.E / nucleon | in Urdu/Hindi | class 12 physics | physics ka safar
Binding energy per nucleon | Nuclei class 12 |
21. Binding energy per nucleon, Nuclear fission and fusion | Pledge 2023 | Nuclei | CBSE | NCERT
Binding Energy Per Nucleon
Calculating Binding Energy Per Nucleon
complete concept and formula sheet tricks binding energy per nucleon and its variation with mass
|| Binding Energy Per Nucleon and Mass Number || binding energy curve ||
Binding Energy Per Nucleon Versus Mass Number Curve, Features & Conclusion, Class 12 Term 2 2022
Q 20 Binding energy per nucleon versus mass number curve for nuclei is shown in the figure . W, X, Y
Binding Energy per Nucleon
Binding energy per nucleon [IB Physics SL/HL]
Binding Energy per Nucleon Curve #07 | Nuclear Chemistry
Binding energy per nucleon AQA Alevel Physics
Binding energy per nucleon for C-14. Carbon 14 mass defect binding energy per nucleon calculation.
Binding Energy Per Nucleon And Packing Fraction,relation between binding energy and packing fraction
Комментарии
 0:19:53
0:19:53
 0:05:57
0:05:57
 0:11:50
0:11:50
 0:13:57
0:13:57
 0:06:08
0:06:08
 0:03:21
0:03:21
 0:01:00
0:01:00
 0:36:29
0:36:29
 0:03:46
0:03:46
 0:18:06
0:18:06
 0:01:01
0:01:01
 0:17:07
0:17:07
 0:00:56
0:00:56
 0:06:28
0:06:28
 0:00:55
0:00:55
 0:13:59
0:13:59
 0:10:33
0:10:33
 0:04:45
0:04:45
 0:06:08
0:06:08
 0:07:03
0:07:03
 0:08:32
0:08:32
 0:11:55
0:11:55
 0:04:26
0:04:26
 0:12:03
0:12:03