filmov
tv
Nuclear Binding Energy Per Nucleon & Mass Defect Problems - Nuclear Chemistry

Показать описание
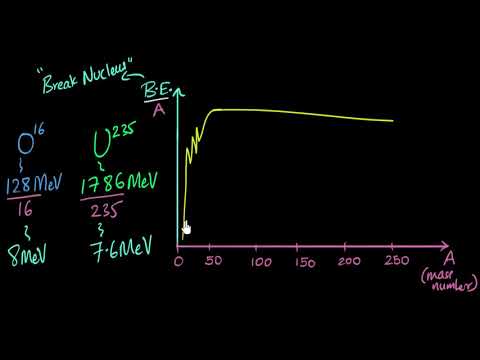
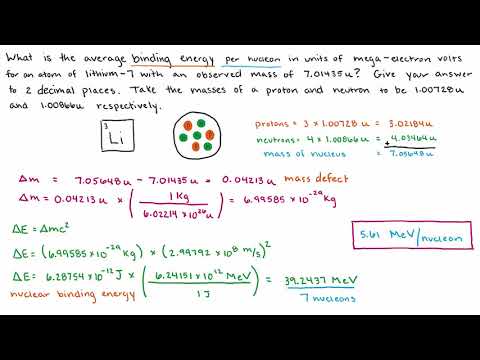
This nuclear chemistry video tutorial explains how to calculate the nuclear binding energy per nucleon for an isotope as well as the mass defect. The mass defect is the difference between the mass of the nucleus and the mass of the nucleons that make up the nucleus such as the protons and neutrons. The change in energy is equal to the mass defect times the square of the speed of light. This video explains how to convert joules into MeV or mega electron volts. This video contains plenty of examples and practice problems.
How To Balance Nuclear Equations:
Alpha, Beta, & Gamma Decay:
Half Life Chemistry Problems:
Carbon-14 Dating Problems:
___________________________________
Nuclear Binding Energy & Mass Defect:
Nuclear Chemistry & Radioactive Decay:
General Chemistry 2 Final Exam Review:
SAT Chemistry Subject Test Review:
____________________________________
Coordinate Covalent Bond:
Complex Ions & Ligands:
Naming Coordination Compounds:
Beer Lambert's Law:
Crystal Field Theory:
___________________________________
ACT Math Practice Test:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
How To Balance Nuclear Equations:
Alpha, Beta, & Gamma Decay:
Half Life Chemistry Problems:
Carbon-14 Dating Problems:
___________________________________
Nuclear Binding Energy & Mass Defect:
Nuclear Chemistry & Radioactive Decay:
General Chemistry 2 Final Exam Review:
SAT Chemistry Subject Test Review:
____________________________________
Coordinate Covalent Bond:
Complex Ions & Ligands:
Naming Coordination Compounds:
Beer Lambert's Law:
Crystal Field Theory:
___________________________________
ACT Math Practice Test:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
 0:19:53
0:19:53
 0:11:50
0:11:50
 0:13:57
0:13:57
 0:03:21
0:03:21
 0:11:38
0:11:38
 0:08:46
0:08:46
 0:20:42
0:20:42
 0:22:49
0:22:49
 0:05:57
0:05:57
 0:07:03
0:07:03
 0:06:08
0:06:08
 0:16:33
0:16:33
 0:08:42
0:08:42
 0:04:19
0:04:19
 0:03:46
0:03:46
 0:08:39
0:08:39
 0:11:55
0:11:55
 0:07:22
0:07:22
 0:03:01
0:03:01
 0:02:36
0:02:36
 0:23:01
0:23:01
 0:03:44
0:03:44
 0:02:26
0:02:26
 0:09:29
0:09:29