filmov
tv
Calculating the Moles of an Element in a Compound

Показать описание
Follow us:
Q1. Propyl acetate, C5H10O2, gives the odor and taste to pears. How many moles of C are present in 1.50 moles of propyl acetate?
Q2. How many moles of propyl acetate, C5H10O2, contain 0.480 mole of O?
Step 1 State the given and needed quantities.
Step 2 Write a plan to convert moles of compound to moles of an element.
Step 3 Write equalities and conversion factors using subscripts.
Step 4 Set up the problem to calculate the moles of an element.
GCSE Chemistry - The Mole (Higher Tier) #25
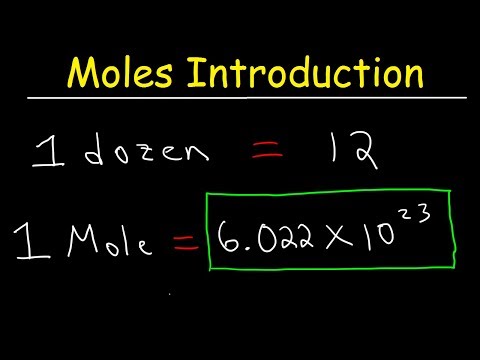
Introduction to Moles
Worked example: Calculating molar mass and number of moles | AP Chemistry | Khan Academy
Mole Conversions Made Easy: How to Convert Between Grams and Moles
How many moles are in 27.0 g of H2O ?
GCSE Chemistry - Moles, Concentration & Volume Calculations #29
Calculating the Moles of an Element in a Compound
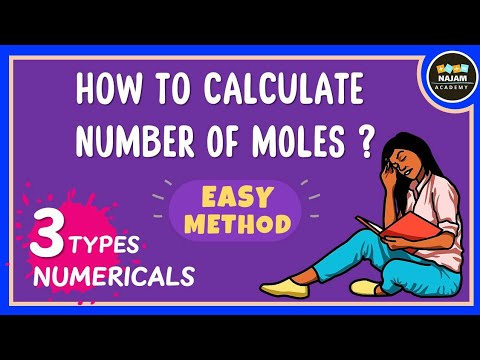
How to calculate the number of moles? Chemistry
Stoichiometry and mole concept | Finding number of moles | well explained
Very Common Mole Questions
How to Calculate Moles?
Stoichiometry Mole to Mole Conversions - Molar Ratio Practice Problems
How to Use a Mole to Mole Ratio | How to Pass Chemistry
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
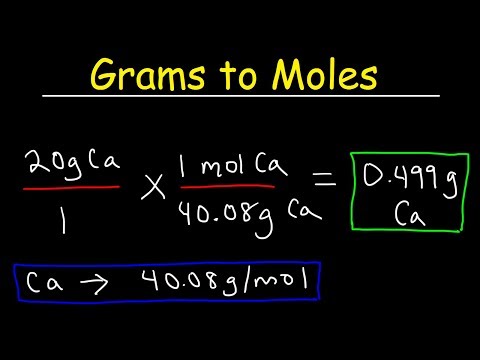
Converting Between Grams and Moles
Calculating the number of Moles in a sample
GCSE Chemistry - Moles and Mass Calculations
Moles In Equations | Chemical Calculations | Chemistry | FuseSchool
Convert Molar Mass to Moles (2021)
How to calculate number of moles|| chemistry
The Mole: Avogadro's Number and Stoichiometry
Introduction to Moles
How To Convert Grams To Moles - VERY EASY!
Calculating Moles - Chemistry Class
Комментарии
 0:04:29
0:04:29
 0:05:16
0:05:16
 0:05:49
0:05:49
 0:07:25
0:07:25
 0:03:14
0:03:14
 0:06:04
0:06:04
 0:03:05
0:03:05
 0:05:29
0:05:29
 0:46:15
0:46:15
 0:10:12
0:10:12
 0:00:29
0:00:29
 0:12:11
0:12:11
 0:02:31
0:02:31
 0:25:16
0:25:16
 0:10:47
0:10:47
 0:05:46
0:05:46
 0:11:03
0:11:03
 0:05:02
0:05:02
 0:01:25
0:01:25
 0:03:16
0:03:16
 0:06:06
0:06:06
 0:10:50
0:10:50
 0:13:17
0:13:17
 0:00:42
0:00:42