filmov
tv
Compare the relative stability of the following species and indicate their magnetic properties.....

Показать описание
NCERT Problem 4.36 Page no. 135
Chemical Bonding and Molecular Structure
Compare the relative stability of the following species and indicate their magnetic properties O2 , O2+ , O2- (superoxide), O22- (peroxide).
Chemical Bonding and Molecular Structure
Compare the relative stability of the following species and indicate their magnetic properties O2 , O2+ , O2- (superoxide), O22- (peroxide).
Compare the relative stability of the following species and indicate their magnetic properties.....
How to compare relative stability of alkenes
Compare the relative stability of the following species and indicate their magneticproperties;
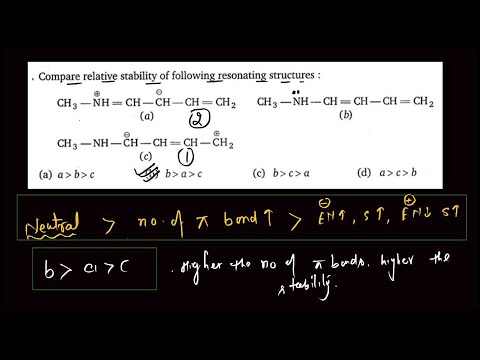
Compare the relative stability of following resonating structures
Compare the relative stability of the following species and indicate their magnetic properties:
Compare the relative stability of the following species and indicate their magnetic properties: ...
Comparison of relative stability of O2,O2-,O2+ and peroxide ion.unit-4/+1CHEMISTRY/chemical bonding.
Compare the relative stability of the following species and indicate their magnetic properties: ...
Compare relative stability of the following resonating structures. ....
Compare relative stability of following carbocation : (i) (ii) (iii) (A) \( \mathrm{i}\mathrm{ii...
Compare relative stability of the following resonating structures. (p) (q) (r) - (a) \( \mathrm{...
Compare relative stability of following resonating structure?
Compare relative stability of following resonating structure: \( \longleftrightarrow \) \( \long...
Carbocation Stability - Hyperconjugation, Inductive Effect & Resonance Structures
Class-11(Unit-4) Q-36 compare the relative stability of oxide,peroxide&superoxide #shzclasses#nc...
Compare relative stability of the following resonating strucrtrue.
Compare relative stability of following resonating structures?
Compare the relative stabilities and magnetic behaviour of the following species (a) `O_(2)^(Θ)`
Stability of Resonance structures Organic Chemistry | IIT JEE & NEET | Vineet Khatri | ATP STAR ...
The relative stability of the compounds given below is in the order.\n....
Evaluating Relative Stability of Chair Conformers
The Relative Stability and Comparison of Alkene Isomers : Cis/Trans, Hyperconjugation, and More
Relative Stability Analysis
Stability of Carbocations
Комментарии
 0:10:24
0:10:24
 0:02:26
0:02:26
 0:30:16
0:30:16
 0:02:37
0:02:37
 0:05:33
0:05:33
 0:05:32
0:05:32
 0:13:05
0:13:05
 0:05:33
0:05:33
 0:02:25
0:02:25
 0:02:39
0:02:39
 0:03:55
0:03:55
 0:04:17
0:04:17
 0:05:36
0:05:36
 0:11:33
0:11:33
 0:09:19
0:09:19
 0:02:15
0:02:15
 0:04:36
0:04:36
 0:07:47
0:07:47
 0:09:23
0:09:23
 0:03:19
0:03:19
 0:09:04
0:09:04
 0:09:24
0:09:24
 0:55:17
0:55:17
 0:15:14
0:15:14