filmov
tv
Carbocation Stability - Hyperconjugation, Inductive Effect & Resonance Structures
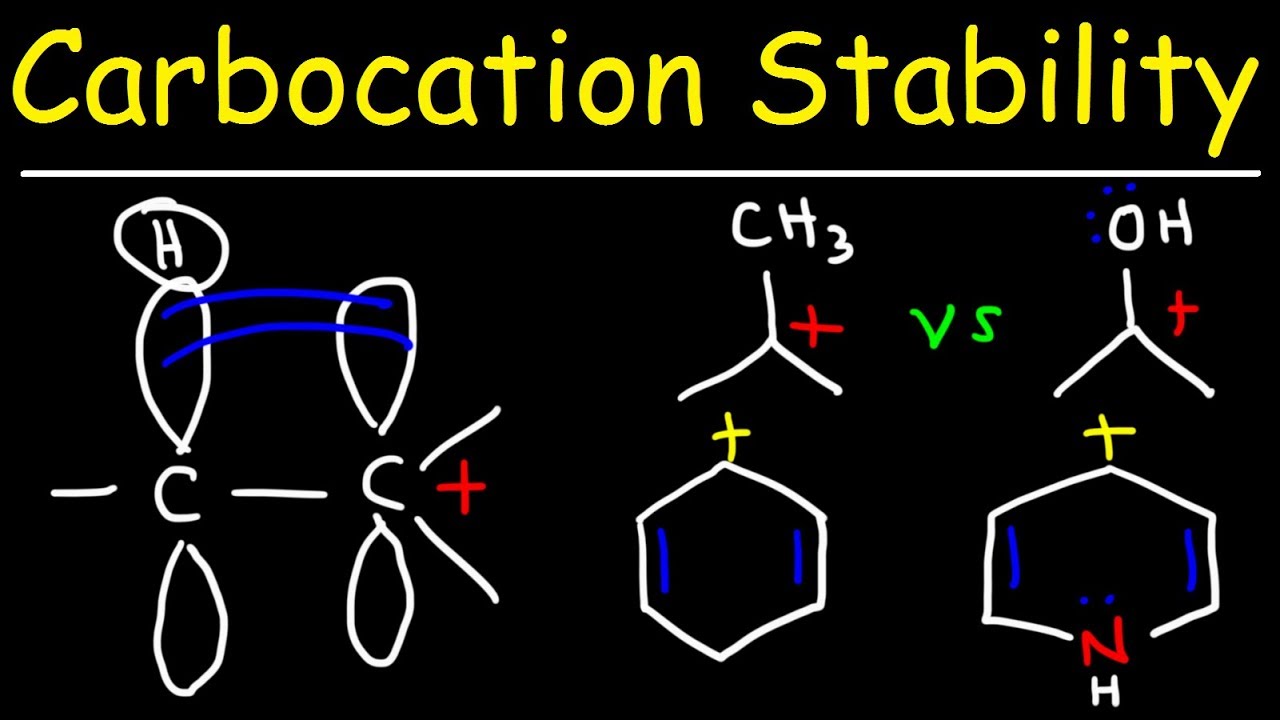
Показать описание
This organic chemistry video tutorial provides a basic introduction into carbocation stability. It discusses hyperconjugation and the inductive effect of electron donating groups and electron withdrawing groups. It also discusses carbocation stability using resonance structures.
Organic Chemistry - Video Lessons:
_________________________________
Stereochemistry R/S Configuration:
Optical Activity & Specific Rotation:
SN1, SN2, E1, E2 Reaction Mechanisms:
SN2 Reaction Mechanisms:
SN2 - Test Question:
_______________________________
SN1 Reaction Mechanisms:
Carbanion Stability:
Protic Vs Aprotic Solvents:
E1 Ring Expansion:
E2 - Test Question:
________________________________
E2 Stereochemistry - Newman Projections:
SN1, SN2, E1, E2 - Practice Test:
Organic Chemistry PDF Worksheets:
Organic Chemistry 1 Exam 2 Playlist:
Full-Length Videos and Worksheets:
Organic Chemistry - Video Lessons:
_________________________________
Stereochemistry R/S Configuration:
Optical Activity & Specific Rotation:
SN1, SN2, E1, E2 Reaction Mechanisms:
SN2 Reaction Mechanisms:
SN2 - Test Question:
_______________________________
SN1 Reaction Mechanisms:
Carbanion Stability:
Protic Vs Aprotic Solvents:
E1 Ring Expansion:
E2 - Test Question:
________________________________
E2 Stereochemistry - Newman Projections:
SN1, SN2, E1, E2 - Practice Test:
Organic Chemistry PDF Worksheets:
Organic Chemistry 1 Exam 2 Playlist:
Full-Length Videos and Worksheets:
Комментарии
 0:11:33
0:11:33
 0:06:23
0:06:23
 0:03:20
0:03:20
 0:11:17
0:11:17
 0:06:33
0:06:33
 0:09:37
0:09:37
 0:04:46
0:04:46
 0:03:33
0:03:33
 0:25:31
0:25:31
 0:02:07
0:02:07
 0:44:14
0:44:14
 0:09:02
0:09:02
 0:09:56
0:09:56
 0:35:17
0:35:17
 0:12:56
0:12:56
 0:05:51
0:05:51
 0:14:54
0:14:54
 0:11:20
0:11:20
 0:05:39
0:05:39
 0:00:37
0:00:37
 1:02:00
1:02:00
 0:13:14
0:13:14
 0:13:54
0:13:54
 0:49:33
0:49:33