filmov
tv
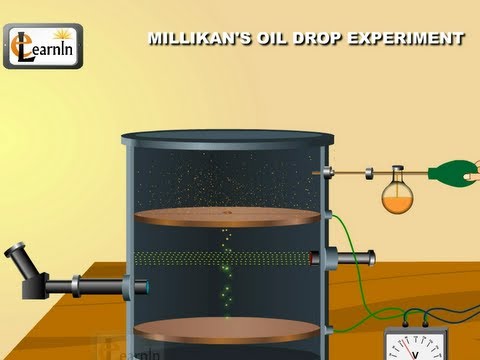
Millikan's oil drop experiment to determine charge of an electron - Chemistry

Показать описание
This chemistry video for Grade 10-11 students demonstrates R. A. Millikan's oil drop experiment to calculate the charge of an electron.
Millikan's oil drop experiment to determine charge of an electron - Chemistry
Charge of an Electron: Millikan's Oil Drop Experiment
Millikan Oil Drop Experiment Animation
Millikan oil-drop experiment || structure of atom || class 11 || VK chemistry lab || 👍👍👍.............
STEM Experiment: Millikan Oil Drop
Measurement of charge on Electron || Millikon's oil droplet method || 11th class chemistry | ch...
Millikans|Oil|Drop|Experiment|Physics 12|Tamil|MurugaMP
The Millikan Experiment
Millikan's Oil Drop Experiment - A Level Physics
Millikan's oil drop experiment explained
Millikan oil drop experiment animation || Charge on electron by millikan's method class 12
Millikan oil drop experiment animation | SWAJ Foundation #electrostatics #education #swaj #animation
millikan's oil drop experiment| Class11 Chapter 2 #yourtutor #shorts #neet #jee
| RT PHYSICS | oil - drop experiment by Robert millikan 😲😲😲#physics #viral #youtube #trending...
Millikan's Oil Drop Experiment to Determine Charge of an Electron | Millikan's Oil Drop Ex...
The Millikan oil drop experiment explained by Miguel (Class of 2017)
Millikan Oil Drop Experiment | Physical Chemistry | NEET JEE | ATP STAR
Millikan's oil drop experiment | Chemistry | #shorts #iitjee #jee #neet #chemistry
Millikans oil drop experiment..... subscribe for more videos
Millikan’s Oil Drop experiment || class 12 modern physics || chapter-Electrons
Parallel Plates & Millikan's Oil Drop Experiment
🔴 Millikan's Oil Drop Experiment || in HINDI
Charge on an electron by Millikan's method | 12th class physics |Millikan's oil drop exper...
Millikan's Oil Drop Experiment // HSC Physics
Комментарии
 0:01:59
0:01:59
 0:12:53
0:12:53
 0:02:03
0:02:03
 0:00:16
0:00:16
 0:03:23
0:03:23
 0:28:23
0:28:23
 0:22:30
0:22:30
 0:00:44
0:00:44
 0:11:44
0:11:44
 0:18:53
0:18:53
 0:00:44
0:00:44
 0:00:59
0:00:59
 0:00:59
0:00:59
 0:00:16
0:00:16
 0:13:53
0:13:53
 0:04:05
0:04:05
 0:13:31
0:13:31
 0:00:33
0:00:33
 0:00:16
0:00:16
 0:15:19
0:15:19
 0:09:47
0:09:47
 0:14:47
0:14:47
 0:32:47
0:32:47
 0:07:16
0:07:16