filmov
tv
Electrochemical Cells - Redox Equilibria - A2 Chemistry Edexcel - Dr Hanaa Assil

Показать описание
Use of reduction potentials to determine overall cell potentials in electrochemical cells, and use of hydrogen fuel cells
Electrochemical Cells - Redox Equilibria - A2 Chemistry Edexcel - Dr Hanaa Assil
Electrochemical Cell | Redox Equilibrium
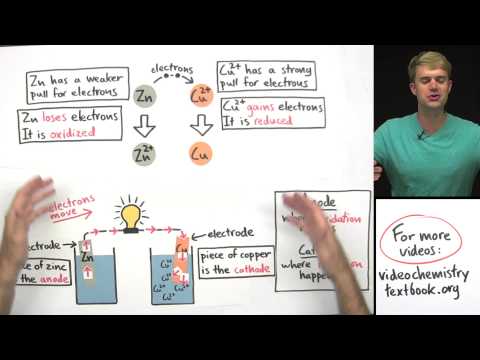
Electrochemistry: Crash Course Chemistry #36
Introduction to electrolysis | Redox reactions and electrochemistry | Chemistry | Khan Academy
Introduction to Galvanic Cells & Voltaic Cells
Electrochemistry
25. Oxidation-Reduction and Electrochemical Cells
Redox Equilibria - Electrochemical Cells & Fuel Cells - AQA A2 Chemistry - Unit 5 - 3.5.3
Redox Equilibria - Electrochemical Series - AQA A2 Chemistry - Unit 5 - 3.5.3
KAC32.5 - Electrochemistry: Electrochemical Cells
Electrochemical Series Explained || Electrode Potentials || Redox Equilibrium || Cells summary
Voltaic cell | How does it work?
KAC32.7 - Electrochemistry: Effect of Concentration on Cell Potentials
Introduction to Electrochemistry
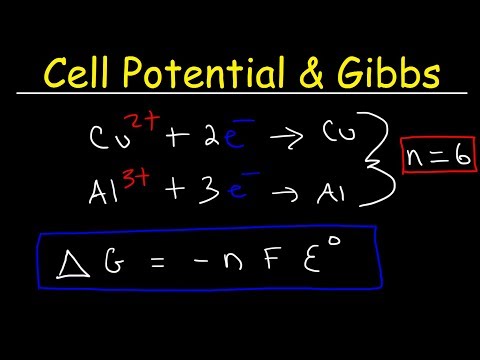
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems
Electrode Potentials & Half Cells | A-level Chemistry | OCR, AQA, Edexcel
16.2-Electrochemical Cells
AQA 1.11 Electrode Potentials and Electrochemical Cells REVISION
Cell Potential Problems - Electrochemistry
Free energy and cell potential | Redox reactions and electrochemistry | Chemistry | Khan Academy
Super-corroding Galvanic Cell used to Heat Soldier’s Meals!
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy
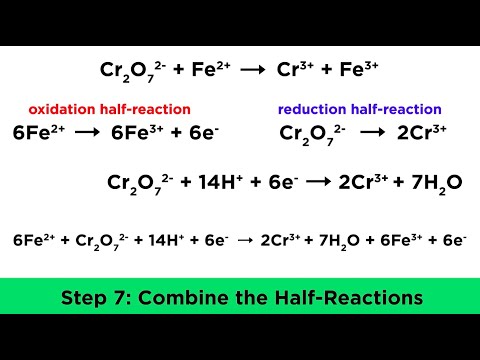
Balancing Redox Reactions in Acidic and Basic Conditions
Calculating Standard Potentials for Electrochemical Cells (Question 1)
Комментарии
 0:47:45
0:47:45
 0:04:32
0:04:32
 0:09:04
0:09:04
 0:06:55
0:06:55
 0:27:42
0:27:42
 0:06:21
0:06:21
 0:53:08
0:53:08
 0:04:34
0:04:34
 0:01:22
0:01:22
 0:05:14
0:05:14
 0:07:23
0:07:23
 0:04:10
0:04:10
 0:07:59
0:07:59
 0:16:37
0:16:37
 0:11:02
0:11:02
 0:19:57
0:19:57
 0:10:24
0:10:24
 0:51:27
0:51:27
 0:10:56
0:10:56
 0:08:14
0:08:14
 0:00:33
0:00:33
 0:09:10
0:09:10
 0:07:31
0:07:31
 0:04:14
0:04:14