filmov
tv
Relative atomic mass and standard atomic weight

Показать описание
----------------------------------------------------
An easy to understand chemistry tutorial, aimed to meet the A-level (grade 11 / 12) chemistry requirements.
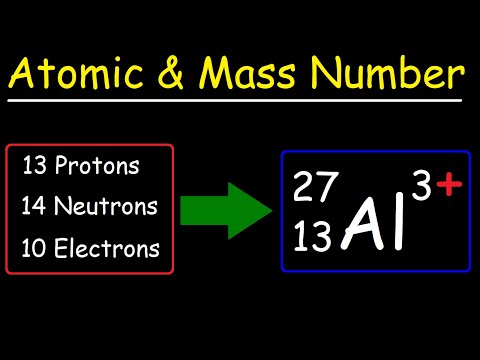
The atomic mass of an atom is the mass of that one atom. Different isotopes will have different atomic masses - so how do we know what the average atomic mass of a chemical element is?
We look at the relative atomic mass. This can sometimes be referred to as the atomic weight of a chemical element (old terminology - no longer used). To calculate the relative atomic mass of an element we need to know three things:
- its different naturally occurring isotopes
- the atomic mass of each of those isotopes
- the abundance of each of those isotopes in a sample
Once you have this information you can calculate the relative atomic mass by (carbon example in video):
- multiplying each isotope's atomic mass by its relative abundance
- adding these multiplications together
- dividing the result by the number of atoms in the sample.
Take carbon for example. In a naturally occurring sample of 100 carbon atoms you'd have two isotopes - carbon-12 (12 u) and carbon-13 (13.003 u).
Ninety-nine atoms in the sample would be carbon-12, and one would be carbon-13. Its relative atomic mass or atomic weight would be:
((12.000 u x 99) + (13.003 x 1))/100 = 12.010
To end this video we introduce you to one more term: standard atomic weight.
The relative atomic mass of a chemical element varies depending on the abundance of isotopes found in a sample. Sometimes different samples will contain different proportions of isotopes. For example, a sample from the ocean floor may be quite different to a sample from the top of mount Everest or even from outer space.
While this can be useful, in helping us identify where samples are from etc. it's also useful to have a standard, for example, for samples that we use in the lab. And that's exactly what the standard atomic weight - the mass based on a 'normal' sample. It's the value that you'll see quoted in periodic tables.
You can turn the subtitles / captions on and off as you please, using the button in the bottom right hand corner.
----------------------------------------------------
An easy to understand chemistry tutorial, aimed to meet the A-level (grade 11 / 12) chemistry requirements.
The atomic mass of an atom is the mass of that one atom. Different isotopes will have different atomic masses - so how do we know what the average atomic mass of a chemical element is?
We look at the relative atomic mass. This can sometimes be referred to as the atomic weight of a chemical element (old terminology - no longer used). To calculate the relative atomic mass of an element we need to know three things:
- its different naturally occurring isotopes
- the atomic mass of each of those isotopes
- the abundance of each of those isotopes in a sample
Once you have this information you can calculate the relative atomic mass by (carbon example in video):
- multiplying each isotope's atomic mass by its relative abundance
- adding these multiplications together
- dividing the result by the number of atoms in the sample.
Take carbon for example. In a naturally occurring sample of 100 carbon atoms you'd have two isotopes - carbon-12 (12 u) and carbon-13 (13.003 u).
Ninety-nine atoms in the sample would be carbon-12, and one would be carbon-13. Its relative atomic mass or atomic weight would be:
((12.000 u x 99) + (13.003 x 1))/100 = 12.010
To end this video we introduce you to one more term: standard atomic weight.
The relative atomic mass of a chemical element varies depending on the abundance of isotopes found in a sample. Sometimes different samples will contain different proportions of isotopes. For example, a sample from the ocean floor may be quite different to a sample from the top of mount Everest or even from outer space.
While this can be useful, in helping us identify where samples are from etc. it's also useful to have a standard, for example, for samples that we use in the lab. And that's exactly what the standard atomic weight - the mass based on a 'normal' sample. It's the value that you'll see quoted in periodic tables.
You can turn the subtitles / captions on and off as you please, using the button in the bottom right hand corner.
----------------------------------------------------
Комментарии
 0:03:48
0:03:48
 0:03:28
0:03:28
 0:04:24
0:04:24
 0:04:16
0:04:16
 0:07:57
0:07:57
 0:02:58
0:02:58
 0:02:54
0:02:54
 0:13:19
0:13:19
 0:19:57
0:19:57
 0:02:41
0:02:41
 0:11:45
0:11:45
 0:04:56
0:04:56
 0:01:56
0:01:56
 0:08:35
0:08:35
 0:00:06
0:00:06
 0:11:41
0:11:41
 0:08:14
0:08:14
 0:08:05
0:08:05
 0:00:20
0:00:20
 0:00:48
0:00:48
 0:01:29
0:01:29
 0:00:34
0:00:34
 0:09:49
0:09:49
 0:00:54
0:00:54