filmov
tv
Effect of Temperature on Elimination and Substitution Reactions
Показать описание
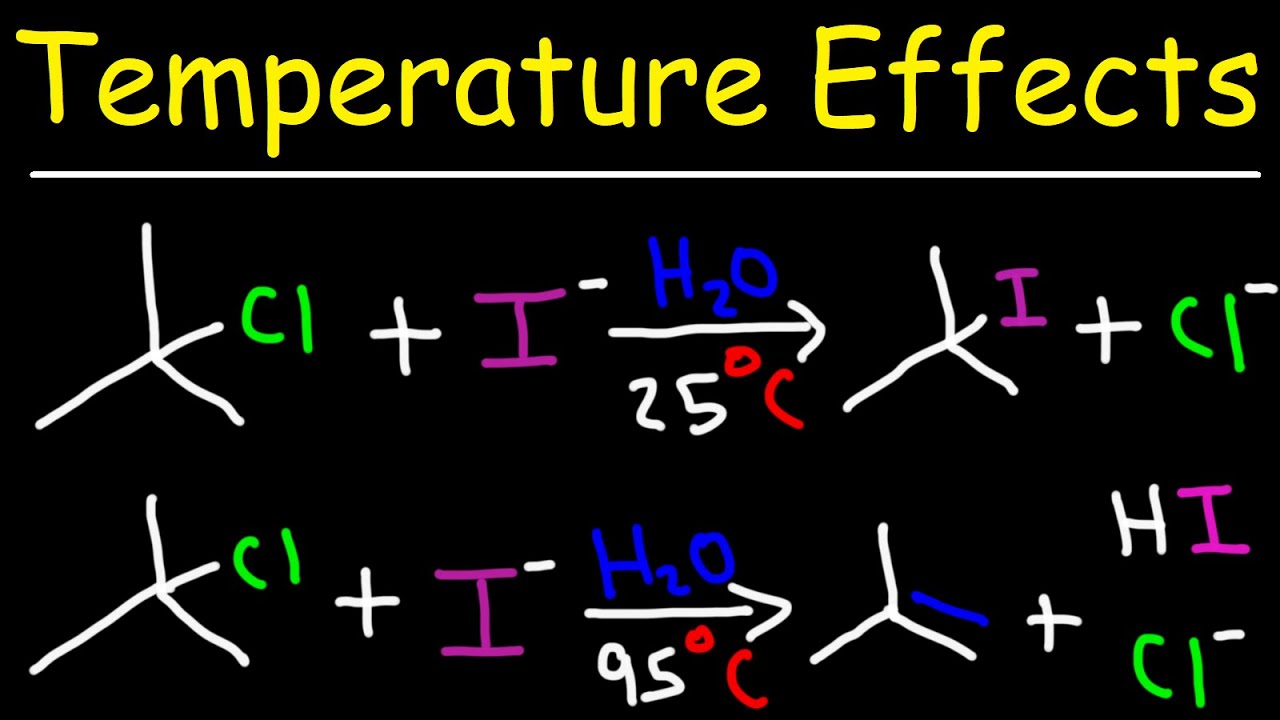
This organic chemistry video tutorial discusses the effect of temperature on elimination and substitution reactions. It explains why E1 reactions are favored over SN1 reactions at high temperatures.
SN1 SN2 E1 E2 - Practice Test Review:
Download The Practice Test - 77 Questions:
Organic Chemistry 1 Final Exam - 6 Hour Review:
Patreon Membership Link:
SN1 SN2 E1 E2 - Practice Test Review:
Download The Practice Test - 77 Questions:
Organic Chemistry 1 Final Exam - 6 Hour Review:
Patreon Membership Link:
Effect of Temperature on Elimination and Substitution Reactions
Explaining Why High Temperature Favors Elimination (E1, E2) Rxns
Ch#17 |Lec#5 | substitution Versus Elimination Reactions Of Alkyl Halides + Factors,
12th Class Chemistry, Effect of Temperature on SN vs Elimination, FSc 2nd year
Elimination Reactions Are Favored by Heat
Reactivity of Elimination reaction Effect of substrate,base, solvent,leaving group ,temperature
Choosing Between SN1/SN2/E1/E2 Mechanisms
Heat in Substitution vs Elimination
Impact of Materials, Transducers & Contemporary Analytics On Design of Next-Gen Gas and Bio Sens...
Factors Effecting E1 & E2 Elimination reactions| Temperature| Substrate|AlkylHalides| base| solv...
Unimolecular Elimination (E1) Reactions
Elimination vs Substitution
An Animated Explanation of Elimination
HIGH TEMPERATURE REASON IN ELIMINATION REACTION 😍
Ch 8 - Part 4 - Kinetics of Substitution and Elimination Reactions
Effects of medium, base , leaving group and substrate structure in elimination reactions
Substitution versus elimination
Elimination reactions - examples
Substitution vs elimination reactions, factors effecting substitution or elimination product.part 3
factors affecting elimination Reactions
The Effect of the solvent and temperature on the Elimination Reactions
Elimination Reactions
E2 Elimination Reaction - Mechanism, Zaitsev and Hoffman, E and Z, Beta Hydrogen- Organic Chemistry
Organic Chemistry 1: Chapter 7 - Substitution and Elimination Reactions (Part 1/5)
Комментарии
 0:10:53
0:10:53
 0:10:15
0:10:15
 0:19:24
0:19:24
 0:03:15
0:03:15
 0:07:42
0:07:42
 0:30:21
0:30:21
 0:18:52
0:18:52
 0:05:27
0:05:27
 1:31:01
1:31:01
 0:11:05
0:11:05
 0:08:49
0:08:49
 0:22:53
0:22:53
 0:07:50
0:07:50
 0:07:14
0:07:14
 0:06:29
0:06:29
 0:10:25
0:10:25
 0:12:06
0:12:06
 0:22:38
0:22:38
 0:11:00
0:11:00
 0:09:34
0:09:34
 0:09:57
0:09:57
 0:33:31
0:33:31
 0:34:16
0:34:16
 0:39:50
0:39:50