filmov
tv
Chemical Thermodynamics 1.6 - Critical Point

Показать описание
Short physical chemistry lecture on the critical properties of non-ideal gases.
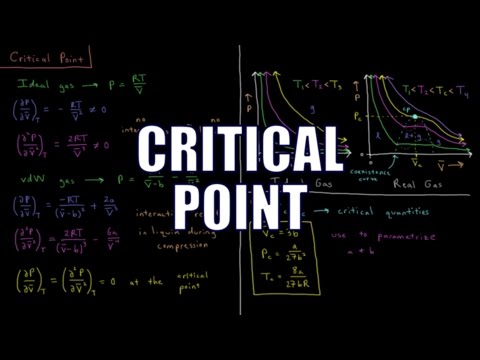
The critical point of a gas is the point where the first and second derivative of pressure with respect to molar volume is zero at the same temperature. All gases have a single unique critical point, which can be used to parametrize the van der Waals equation of state.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
The critical point of a gas is the point where the first and second derivative of pressure with respect to molar volume is zero at the same temperature. All gases have a single unique critical point, which can be used to parametrize the van der Waals equation of state.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
Chemical Thermodynamics 1.6 - Critical Point
Chemical Thermodynamics 1.0 - Gas Properties Review
Chemical Thermodynamics 0.1 - Introduction
Chemical Thermodynamics Review | Chemistry Matters
Boyle’s Law
Chemical Thermodynamics 1.0 - Gas Properties Review (Old Version)
What is Thermodynamics? | Class 11 Physics Explained
Thermodynamics In Just 30 Minutes! | REVISION - Super Quick! JEE & NEET Chemistry | Pahul Sir
Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams
Chemical Thermodynamics Lecture 6 part 1
HONEST NEET JOURNEY IN 12 SECONDS! #neet #neetmotivation
Plus One Chemistry - Thermodynamics | Xylem Plus One
Thermodynamics | CBSE Class 11 Chemistry | Full Chapter-5 in 1️⃣5️⃣ Mins | Rapid Revision Series...
Absolute Zero!🤧 #shorts
The free energy of the liquid surface does the work #shorts #physics
Class 11th Chemistry - Thermodynamics | Thermodynamics Class 11 Chemistry by GlobalShiksha.com
Basic Concepts of Thermodynamics (Animation)
Mastering Class 11 Chemistry Thermodynamics Made notes Easy #neet #chemistry #neetexam
THERMODYNAMICS in 55 Minutes || Full Chapter Revision || Class 11th JEE
Chemical Reaction 🧫🔥।। Easy science experiment 👨🔬।। #ytshorts #viral #shorts #science...
6 Most Important Chapters for NEET Chemistry || #NEET2024 || #NEETPreparation ||@srichaitanyagosala
Liquefaction of gases-chemical thermodynamic 1
+1 Improvement Exam | Chemistry | Chemical Bonding /Thermodynamics | Exam Winner
Chemical thermodynamics, Intro: 3 Laws of TD
Комментарии
 0:07:32
0:07:32
 0:04:34
0:04:34
 0:04:36
0:04:36
 0:06:03
0:06:03
 0:00:15
0:00:15
 0:04:24
0:04:24
 0:00:53
0:00:53
 0:31:05
0:31:05
 0:04:51
0:04:51
 0:51:22
0:51:22
 0:00:12
0:00:12
 1:17:16
1:17:16
 0:15:40
0:15:40
 0:00:46
0:00:46
 0:00:12
0:00:12
 0:23:36
0:23:36
 0:10:57
0:10:57
 0:00:10
0:00:10
 0:55:33
0:55:33
 0:00:23
0:00:23
 0:00:23
0:00:23
 0:08:43
0:08:43
 1:27:22
1:27:22
 0:12:12
0:12:12