filmov
tv
Chemical Kinetics 01 | Rate of Reaction | Class 12th/CUET

Показать описание
📝 For complete notes of Lectures, visit SANKALP 2023 Batch in the Batch Section of PhysicsWallah App/Website.
We are going to cover the topic-Rate of chemical reaction of chapter- Chemical kinetics in this lecture.
0:00 Introduction
1:17 Content covered
2:17 Thermodynamics
5:37 Chemical kinetics
16:13 Types of Rates
27:31 Factors affecting the rate of reaction
29:41 Rate Law
35:25 Unit of Rate constant
41:25 First order reaction
43:07 Effect of concentration on rate
54:10 Order of a reaction
1:04:00 Types of reaction
1:09:47 Molecularity
1:17:38 Questions based on Rate of reaction
1:31:35 Thank You
There are many other similar lectures in upcoming videos for class 12. Stay tuned with NCERT Wallah.
-------------------------------------------------------------------------------------------------------
NOTE: This batch is completely FREE, you just have to click on the "BUY NOW" button for your enrolment.
🔴 Batch Details:-
✅ FREE Course for Class 12th (Board + CUET)
1. This free batch is for the students aiming Class 12th (Board + CUET).
2. The best faculties of India will cover full syllabus of each subject.
3. All the classes will be provided in Recorded form only on our YouTube Channel Ncert Wallah.
4. Class Notes of each lecture will be provided on our PW App in respective batch in PDF format.
5. Practice sheets with text solutions of each lectures will be also provided on App.
6. Weekly Schedule of classes will be posted on Community and announcement section of Batch.
-----------------------------------------------------------------------------------------------------------------------------------------
📌 PHYSICS WALLAH OTHER CHANNELS :
📌 PHYSICS WALLAH SOCIAL MEDIA PROFILES :
#ChemicalKinetics #Chemistry #NCERTWallah #PhysicsWallah #RateOfReaction #Class12 #CUET #ChemicalKineticsClass12 #RateOfReactionClass12 #RateOfReactionChemistry #RateOfReactionCUETChemistry #CUETChemistry #ChemicalKineticsCUET2023 #Class12ChemicalKinetics #CUET2023
Комментарии
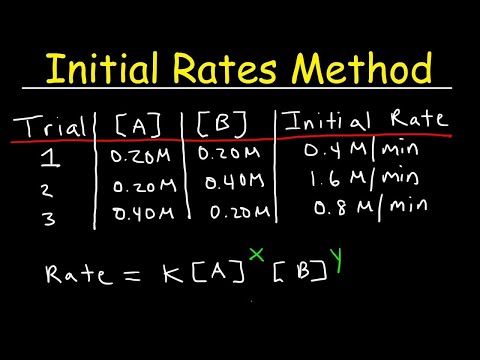 0:34:53
0:34:53
 0:09:10
0:09:10
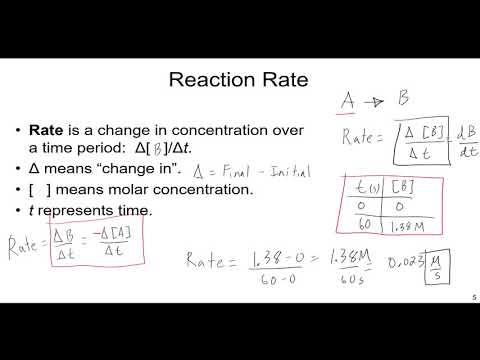 0:25:24
0:25:24
 0:48:46
0:48:46
 0:20:55
0:20:55
 1:07:52
1:07:52
 0:10:39
0:10:39
 1:45:04
1:45:04
 0:51:11
0:51:11
 0:44:29
0:44:29
 1:30:02
1:30:02
 0:23:00
0:23:00
 0:25:20
0:25:20
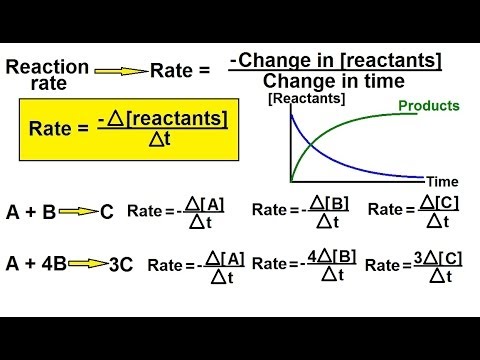 0:05:35
0:05:35
 1:32:11
1:32:11
 0:50:42
0:50:42
 1:07:56
1:07:56
 0:06:23
0:06:23
 0:11:36
0:11:36
 0:00:11
0:00:11
 0:42:56
0:42:56
 2:34:54
2:34:54
 0:09:08
0:09:08
 1:45:25
1:45:25