filmov
tv
Calculation of Standard Electrode potential (E° Cell)-FOR NEET and JEE👍

Показать описание
Cell Potential Problems - Electrochemistry
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy
R3.2.13 Calculating cell potential (HL)
Calculation of Standard Electrode potential (E° Cell)-FOR NEET and JEE👍
Standard Reduction Potentials of Half Reactions - Electrochemistry
19.4 How to Calculate Standard Cell Potential | General Chemistry
Standard Electrode Potential Value | Chemistry
19.1 Standard electrode potential (HL)
Electrochemistry
Using standard reduction potentials to calculate a cell’s potential
Electrochemistry- Standard Electrode Potential
Worked example: Calculating E° using standard reduction potentials | AP Chemistry | Khan Academy
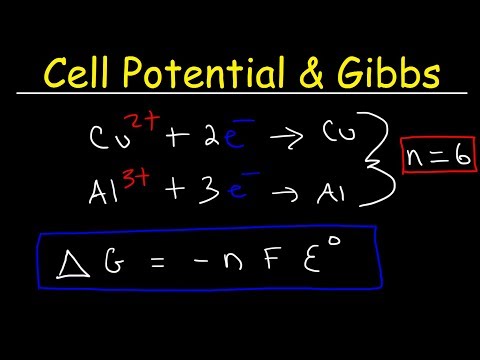
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems
Electrochemistry | Calculations on Electrode Potential | Chemistry Tutorials
R3.2.13 Calculate cell potentials using standard electrode potentials [HL IB Chemistry]
19.1 Standard electrode potential (HL)
Electrochem Eng L02-08 Notes for standard electrode potential
Electrochemistry / class 12 / numerical / calculation of standard electrode potential / free energy
Calculating Standard Potentials for Electrochemical Cells (Question 1)
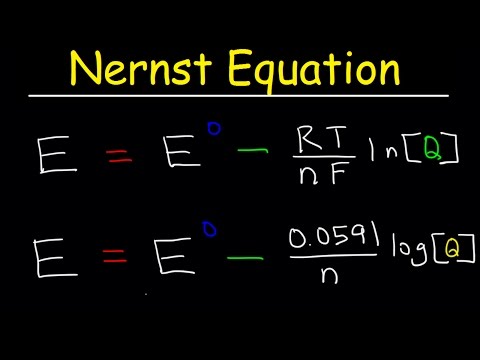
Nernst Equation Explained, Electrochemistry, Example Problems, pH, Chemistry, Galvanic Cell
How to Calculate Standard Cell Potential and Voltage Part 1: Examples & Practice Problems
standard electrode potential (electrochemistry)❣️
Standard Electrode Potentials | U3 | ATAR Chemistry QCE (updated)
Standard Hydrogen Electrode Summary for A-level Chemistry (standard hydrogen half cell)
Комментарии
 0:10:56
0:10:56
 0:09:10
0:09:10
 0:05:27
0:05:27
 0:02:35
0:02:35
 0:11:48
0:11:48
 0:27:14
0:27:14
 0:00:19
0:00:19
 0:04:28
0:04:28
 0:06:21
0:06:21
 0:05:42
0:05:42
 0:00:47
0:00:47
 0:05:21
0:05:21
 0:11:02
0:11:02
 0:10:04
0:10:04
 0:01:33
0:01:33
 0:05:34
0:05:34
 0:10:14
0:10:14
 0:07:56
0:07:56
 0:04:14
0:04:14
 0:30:53
0:30:53
 0:05:55
0:05:55
 0:00:16
0:00:16
 0:02:38
0:02:38
 0:05:19
0:05:19