filmov
tv
Standard Reduction Potentials of Half Reactions - Electrochemistry

Показать описание
This chemistry video tutorial provides a basic introduction into standard reduction potentials of half reactions. It explains how to identify the strongest oxidizing agent and the strongest reducing agent. Oxidizing agents are found on the left side of the reduction reaction and reducing agents are found on the right side of the reduction. Oxidizing agents have a strong affinity for electrons and reducing agents have a strong desire to give away electrons. This electrochemistry has plenty of questions and practice problems relating to standard reduction potentials.
Intro to Galvanic & Voltaic Cells:
How To Draw Galvanic Cells:
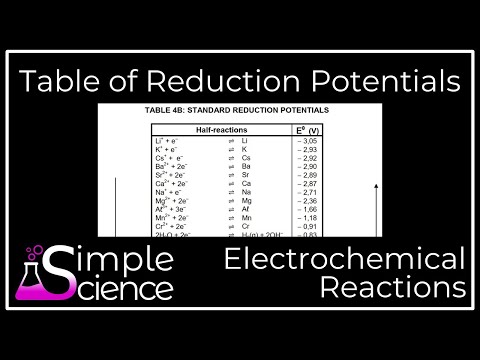
Standard Reduction Potentials:
Cell Potential Problems:
Cell Notation Problems:
___________________________________
Concentration Cells:
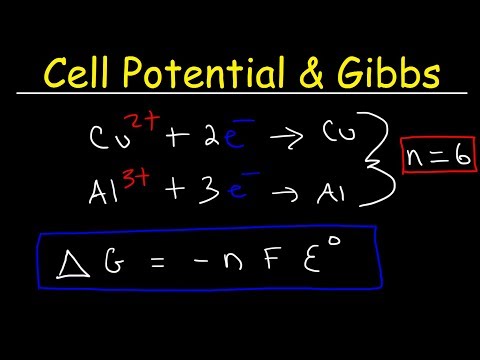
Cell Potential & Gibbs Free Energy:
Cell Potential & Equilibrium K:
Nernst Equation:
Electrolysis of Water:
_____________________________________
Electrolysis of Sodium Chloride:
Electrolysis & Electroplating Problems:
Electrochemistry Practice Problems:
SAT Chemistry Subject Test Review:
Carbon -14 Dating:
Beer Lambert's Law:
______________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Intro to Galvanic & Voltaic Cells:
How To Draw Galvanic Cells:
Standard Reduction Potentials:
Cell Potential Problems:
Cell Notation Problems:
___________________________________
Concentration Cells:
Cell Potential & Gibbs Free Energy:
Cell Potential & Equilibrium K:
Nernst Equation:
Electrolysis of Water:
_____________________________________
Electrolysis of Sodium Chloride:
Electrolysis & Electroplating Problems:
Electrochemistry Practice Problems:
SAT Chemistry Subject Test Review:
Carbon -14 Dating:
Beer Lambert's Law:
______________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
 0:11:48
0:11:48
 0:09:10
0:09:10
 0:04:24
0:04:24
 0:06:21
0:06:21
 0:11:02
0:11:02
 0:05:21
0:05:21
 0:04:42
0:04:42
 0:19:57
0:19:57
 1:34:44
1:34:44
 0:10:59
0:10:59
 0:12:30
0:12:30
 0:09:04
0:09:04
 0:15:46
0:15:46
 0:12:07
0:12:07
 0:10:56
0:10:56
 0:04:28
0:04:28
 0:06:08
0:06:08
 0:06:01
0:06:01
 0:02:28
0:02:28
 0:06:56
0:06:56
 0:05:58
0:05:58
 0:05:34
0:05:34
 0:03:09
0:03:09
 0:10:21
0:10:21