filmov
tv
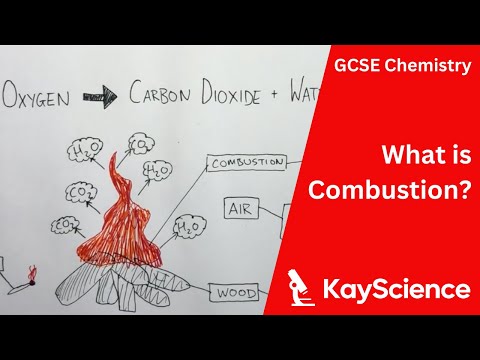
Combustion simplified

Показать описание
1. Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent, typically oxygen. During combustion, the fuel undergoes a chemical change that releases heat and light.
2. The three essential components of combustion are fuel, oxygen, and heat. The fuel provides the carbon and hydrogen that combine with oxygen to form carbon dioxide and water, while the heat provides the activation energy needed to initiate the reaction.
3. Combustion is an exothermic reaction, which means that it releases energy in the form of heat and light. This energy can be harnessed to perform work, such as powering an engine or generating electricity.
4. Combustion can be incomplete, resulting in the formation of toxic gases such as carbon monoxide. To prevent incomplete combustion, it is important to ensure that the fuel and air mixture is properly proportioned and that there is sufficient heat to sustain the reaction.
5. The efficiency of combustion can be improved through the use of technologies such as catalytic converters, which help to convert harmful pollutants into less harmful substances. Additionally, alternative fuels such as biofuels and hydrogen can be used to reduce emissions and improve sustainability.

#sciencefacts #combustion #firefacts #shorts #scienceshorts #homeschool
2. The three essential components of combustion are fuel, oxygen, and heat. The fuel provides the carbon and hydrogen that combine with oxygen to form carbon dioxide and water, while the heat provides the activation energy needed to initiate the reaction.
3. Combustion is an exothermic reaction, which means that it releases energy in the form of heat and light. This energy can be harnessed to perform work, such as powering an engine or generating electricity.
4. Combustion can be incomplete, resulting in the formation of toxic gases such as carbon monoxide. To prevent incomplete combustion, it is important to ensure that the fuel and air mixture is properly proportioned and that there is sufficient heat to sustain the reaction.
5. The efficiency of combustion can be improved through the use of technologies such as catalytic converters, which help to convert harmful pollutants into less harmful substances. Additionally, alternative fuels such as biofuels and hydrogen can be used to reduce emissions and improve sustainability.

#sciencefacts #combustion #firefacts #shorts #scienceshorts #homeschool
 0:02:06
0:02:06
 0:03:56
0:03:56
 0:01:39
0:01:39
 0:00:10
0:00:10
 0:05:17
0:05:17
 0:00:11
0:00:11
 0:00:19
0:00:19
 0:07:55
0:07:55
 0:08:21
0:08:21
 0:03:30
0:03:30
 0:00:11
0:00:11
 0:05:27
0:05:27
 0:00:13
0:00:13
 0:05:42
0:05:42
 0:11:48
0:11:48
 0:04:17
0:04:17
 0:01:24
0:01:24
 0:00:13
0:00:13
 0:05:01
0:05:01
 0:00:09
0:00:09
 0:09:52
0:09:52
 0:01:58
0:01:58
 0:16:42
0:16:42
 0:55:15
0:55:15