filmov
tv
Calculating Average Atomic Mass of an element with isotopes

Показать описание
Calculating Average Atomic Mass of an element with isotopes
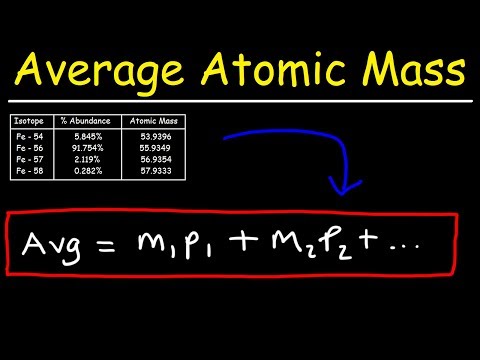
In this video I show you how to use isotopes and their percent abundance and be able to calculate the average atomic mass of an element.
You need both the isotope mass and percent abundance of each to do this.
Example formula:
(Mass 1 * Abundance 1) + (Mass 2 * Abundance 2) + (Mass 3 * Abundance 3)
NOTE: be sure to convert your percent to a decimal for each calculation in the process.
Keywords:
Calculating average atomic mass, isotope abundance, hs chemistry, regents chemistry, how to calculate average atomic mass,
In this video I show you how to use isotopes and their percent abundance and be able to calculate the average atomic mass of an element.
You need both the isotope mass and percent abundance of each to do this.
Example formula:
(Mass 1 * Abundance 1) + (Mass 2 * Abundance 2) + (Mass 3 * Abundance 3)
NOTE: be sure to convert your percent to a decimal for each calculation in the process.
Keywords:
Calculating average atomic mass, isotope abundance, hs chemistry, regents chemistry, how to calculate average atomic mass,
 0:07:19
0:07:19
 0:06:11
0:06:11
 0:08:38
0:08:38
 0:04:26
0:04:26
 0:10:18
0:10:18
 0:06:59
0:06:59
 0:06:15
0:06:15
 0:12:15
0:12:15
 0:23:26
0:23:26
 0:07:16
0:07:16
 0:11:49
0:11:49
 0:10:01
0:10:01
 0:06:41
0:06:41
 0:06:55
0:06:55
 0:03:48
0:03:48
 0:08:23
0:08:23
 0:09:52
0:09:52
 0:04:48
0:04:48
 0:02:39
0:02:39
 0:00:58
0:00:58
 0:10:25
0:10:25
 0:03:47
0:03:47
 0:03:55
0:03:55
 0:03:44
0:03:44