filmov
tv
Metallic Bonding & Properties Tutorial [Now with Animations!] | The Crash Chemistry Academy
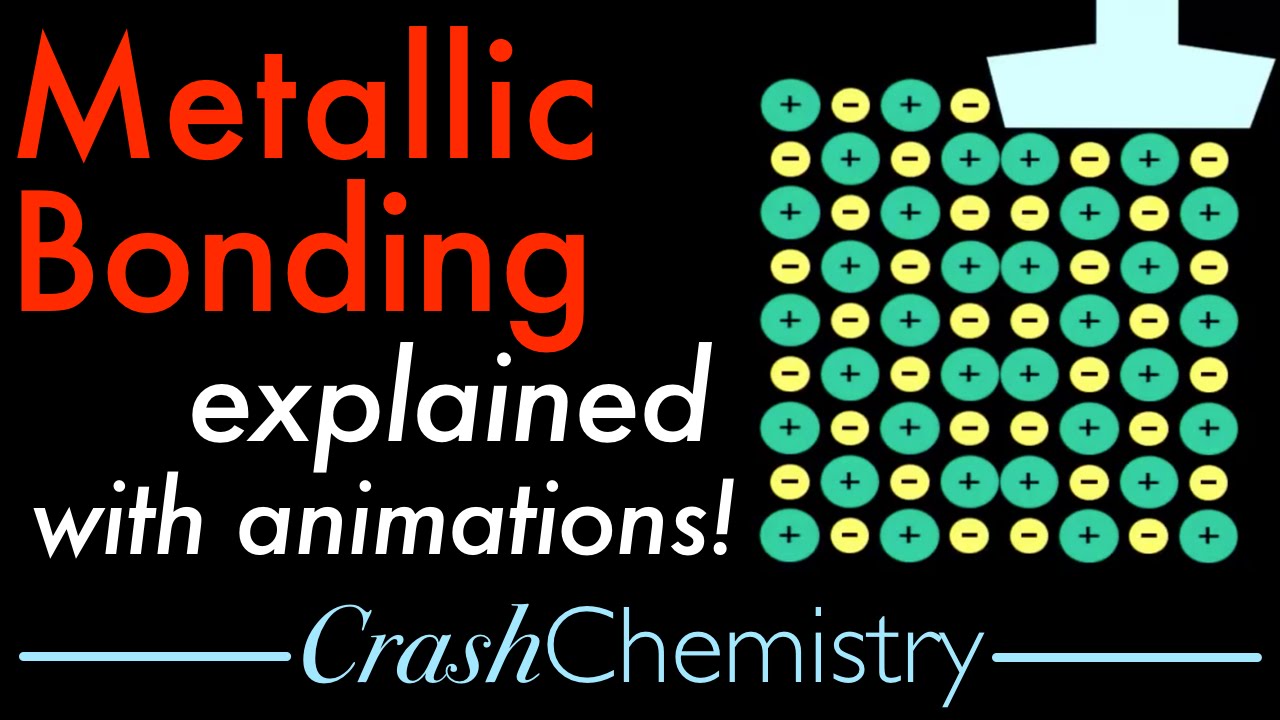
Показать описание
electron sea model of bonding is explained and used to explain metallic properties such as malleability, conductivity, and luster
CC Academy videos are easy 101 crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
Check out our best lessons:
- Solution Stoichiometry Tutorial: How to use Molarity
- Stoichiometry
- Quantum Numbers
- Rutherford's Gold Foil Experiment, Explained
- Covalent Bonding Tutorial: Covalent vs. Ionic bonds
- Metallic Bonding and Metallic Properties Explained: Electron Sea Model
- Effective Nuclear Charge, Shielding, and Periodic Properties
- Electron Configuration Tutorial + How to Derive Configurations from Periodic Table
- Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy
- Metric Prefix Conversions Tutorial
- Gas Law Practice Problems: Boyle's Law, Charles Law, Gay Lussac's, Combined Gas Law
—More on Metallic Bonds | Wikipedia—
"Metallic bonding is the force of attraction between valence electrons and the metal ions. It is the sharing of many detached electrons between many positive ions, where the electrons act as a "glue" giving the substance a definite structure.
The electrons and the positive ions in the metal have a strong attractive force between them. Therefore, metals often have high melting or boiling points. The principle is similar to that of ionic bonds.
The metallic bond causes many of the traits of metals, such as strength, malleability, ductility, luster, conduction of heat and electricity.
Because the electrons move freely, the metal has some electrical conductivity. It allows the energy to pass quickly through the electrons, generating a current. Metals conduct heat for the same reason: the free electrons can transfer the energy at a faster rate than other substances with electrons that are fixed into position. There also are few non-metals which conduct electricity: graphite (because, like metals, it has free electrons), and ionic compounds that are molten or dissolved in water, which have free moving ions.
Metal bonds have at least one valence electron which they do not share with neighboring atoms, and they do not lose electrons to form ions. Instead the outer energy levels (atomic orbitals) of the metal atoms overlap. They are similar to covalent bonds.[4] Not all metals exhibit metallic bonding. For example, the mercurous ion (Hg2+
2) forms covalent metal-metal bonds.
An alloy is a solution of metals."
"Metallic bond." Wikipedia, The Free Encyclopedia. 4 Feb 2016, 17:37 UTC. 27 May 2016, 19:20
CC Academy videos are easy 101 crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
Check out our best lessons:
- Solution Stoichiometry Tutorial: How to use Molarity
- Stoichiometry
- Quantum Numbers
- Rutherford's Gold Foil Experiment, Explained
- Covalent Bonding Tutorial: Covalent vs. Ionic bonds
- Metallic Bonding and Metallic Properties Explained: Electron Sea Model
- Effective Nuclear Charge, Shielding, and Periodic Properties
- Electron Configuration Tutorial + How to Derive Configurations from Periodic Table
- Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy
- Metric Prefix Conversions Tutorial
- Gas Law Practice Problems: Boyle's Law, Charles Law, Gay Lussac's, Combined Gas Law
—More on Metallic Bonds | Wikipedia—
"Metallic bonding is the force of attraction between valence electrons and the metal ions. It is the sharing of many detached electrons between many positive ions, where the electrons act as a "glue" giving the substance a definite structure.
The electrons and the positive ions in the metal have a strong attractive force between them. Therefore, metals often have high melting or boiling points. The principle is similar to that of ionic bonds.
The metallic bond causes many of the traits of metals, such as strength, malleability, ductility, luster, conduction of heat and electricity.
Because the electrons move freely, the metal has some electrical conductivity. It allows the energy to pass quickly through the electrons, generating a current. Metals conduct heat for the same reason: the free electrons can transfer the energy at a faster rate than other substances with electrons that are fixed into position. There also are few non-metals which conduct electricity: graphite (because, like metals, it has free electrons), and ionic compounds that are molten or dissolved in water, which have free moving ions.
Metal bonds have at least one valence electron which they do not share with neighboring atoms, and they do not lose electrons to form ions. Instead the outer energy levels (atomic orbitals) of the metal atoms overlap. They are similar to covalent bonds.[4] Not all metals exhibit metallic bonding. For example, the mercurous ion (Hg2+
2) forms covalent metal-metal bonds.
An alloy is a solution of metals."
"Metallic bond." Wikipedia, The Free Encyclopedia. 4 Feb 2016, 17:37 UTC. 27 May 2016, 19:20
Комментарии
 0:03:31
0:03:31
 0:06:08
0:06:08
 0:10:29
0:10:29
 0:09:10
0:09:10
 0:04:14
0:04:14
 0:05:54
0:05:54
 0:05:25
0:05:25
 0:02:40
0:02:40
 0:01:13
0:01:13
 0:03:20
0:03:20
 0:09:11
0:09:11
 0:03:36
0:03:36
 0:05:32
0:05:32
 0:04:40
0:04:40
 0:05:54
0:05:54
 0:06:44
0:06:44
 0:03:06
0:03:06
 0:11:50
0:11:50
 0:05:42
0:05:42
 0:22:11
0:22:11
 0:03:09
0:03:09
 0:04:28
0:04:28
 0:03:22
0:03:22
 0:16:43
0:16:43