filmov
tv
Metallic Bonding | Chemistry

Показать описание
I will teach you about metallic bonds in chemistry
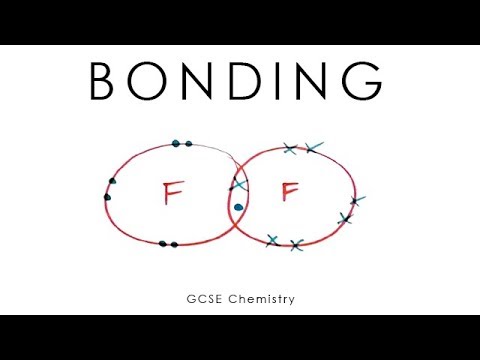
Also, you will learn about the difference between ionic bonding, covalent bonding and metallic bonding.
Q: What are metallic bonds?
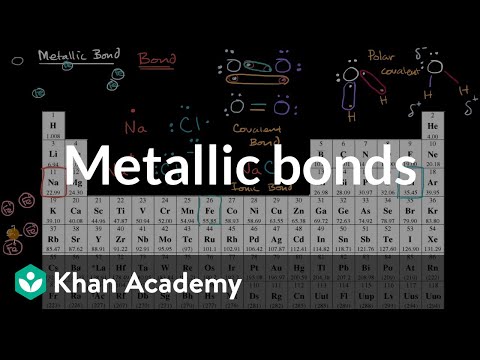
Ans: The chemical bond formed due to electrostatic force between positive ions of metal and the sea of electrons is known metallic bond. Metallic bond exist in all metals.
Metallic bond is a strong chemical bond.
For example, the example of metallic bond is iron sheet, zinic, gold, silver, copper, etc.
Q: What is electron sea theory?
Q: What is the electron sea model of metallic bonding?
Ans: In metallic bonding, atoms neither transfer nor share electrons. Rather, metallic bond is explained on the basis of electron sea theory or electron gas theory which we usually call electron sea model of metallic bonding.
According to electron sea theory, all atoms in metal lattice lose their respective valence electrons and become positive ions. These lost electrons form a sea of electrons or clouds of electrons around the positive ions. Electrostatic force produces between positive ions and sea of electrons which give birth to metallic bonding.
#MetallicBonding
#ElectronSeaModel
#NajamAcademy
Also, you will learn about the difference between ionic bonding, covalent bonding and metallic bonding.
Q: What are metallic bonds?
Ans: The chemical bond formed due to electrostatic force between positive ions of metal and the sea of electrons is known metallic bond. Metallic bond exist in all metals.
Metallic bond is a strong chemical bond.
For example, the example of metallic bond is iron sheet, zinic, gold, silver, copper, etc.
Q: What is electron sea theory?
Q: What is the electron sea model of metallic bonding?
Ans: In metallic bonding, atoms neither transfer nor share electrons. Rather, metallic bond is explained on the basis of electron sea theory or electron gas theory which we usually call electron sea model of metallic bonding.
According to electron sea theory, all atoms in metal lattice lose their respective valence electrons and become positive ions. These lost electrons form a sea of electrons or clouds of electrons around the positive ions. Electrostatic force produces between positive ions and sea of electrons which give birth to metallic bonding.
#MetallicBonding
#ElectronSeaModel
#NajamAcademy
Комментарии
 0:03:31
0:03:31
 0:04:14
0:04:14
 0:10:29
0:10:29
 0:06:08
0:06:08
 0:04:28
0:04:28
 0:03:03
0:03:03
 0:05:54
0:05:54
 0:04:40
0:04:40
 0:19:13
0:19:13
 0:05:42
0:05:42
 0:02:13
0:02:13
 0:00:26
0:00:26
 0:03:00
0:03:00
 0:10:10
0:10:10
 0:11:50
0:11:50
 0:04:41
0:04:41
 0:09:10
0:09:10
 0:05:25
0:05:25
 0:00:27
0:00:27
 0:23:55
0:23:55
 0:01:13
0:01:13
 0:09:11
0:09:11
 0:03:34
0:03:34
 0:00:22
0:00:22