filmov
tv
Making An Instant Drink Cooler Using Lego

Показать описание
See my latest experiments and behind-the-scenes footage!
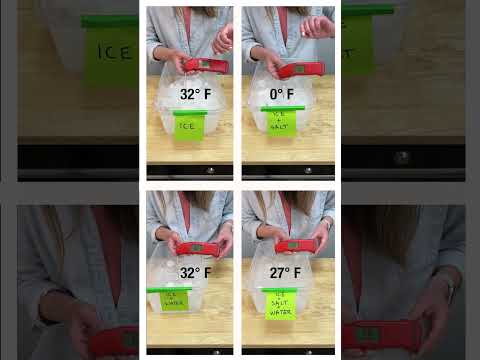
Can we use a combination of Lego and technology to make a gadget that can quickly chill a drink? There's nothing worse than reaching for a refreshing drink, only to realise there's nothing in the fridge. But perhaps it's possible to chill a drink in only a couple minutes!
This invention experiments with Peltier modules which can draw heat from objects, cooling them. So is it possible to draw heat from a drink? And if so, how quickly? We'll use Lego to extract heat from the heatsink via a fan, and Lego will also be used to transport our drink via a peristaltic pump. Let's find out how quickly we can chill our drink!
Can we use a combination of Lego and technology to make a gadget that can quickly chill a drink? There's nothing worse than reaching for a refreshing drink, only to realise there's nothing in the fridge. But perhaps it's possible to chill a drink in only a couple minutes!
This invention experiments with Peltier modules which can draw heat from objects, cooling them. So is it possible to draw heat from a drink? And if so, how quickly? We'll use Lego to extract heat from the heatsink via a fan, and Lego will also be used to transport our drink via a peristaltic pump. Let's find out how quickly we can chill our drink!
Making An Instant Drink Cooler Using Lego
Instant Drink Chiller - How to Make a Cooling Machine
Cup Cooler Instant – Cool Your Drink Instant with No Ice
Drink cool! - how to make cup cooling gadget - Cooler Peltier
I made a Rapid Drink Cooler ❄️
Instant LEGO Drink Cooler (Chills Drinks in 2 Minutes)
DIY Instant Water Cooler | Easy Homemade Cooling System
Thermoelectric Peltier Cooler Build & Freezing Water! #cool #engineering #experiment #electronic...
Refreshing Sweet Sattu Drink Perfect Summer Cooler#SummerDrink #TrendingNow #FoodTrend #ViralRecipe
water chiller #waseem4259 #diy #airconditioner #knowledge_factory
How to Cool Your Drinks FAST! | Everyday Awesome
Water Cooling Dispenser Make Electric Water Cooler From Compressor Homemade Water Dispenser
Chill O Matic Instant Beverage Cooler #shorts
How to make instant water cooler
Cup cooler review: Instagram SMART COOLING CUP, summer beverage cooler [178]
Water Cooling Dispenser Electric Water Cooler Dispenser With 40 litres Tank Instant Cool
How do you make instant cold water | DIY Instant Water cooler /chiller | Peltier Water Cooler/fridge
Peltier Soda Cooler Machine - DIY Drink Dispenser
Instant Summer Cooler || Easy Summer Premix || Homemade With NDS
Making Cooler/Generator with Thermoelectric Device
CHILLED IN SECONDS! INSTANT DRINK COOLER FOR HOT DAYS! #amazonhome #amazon #amazonfinds #summer
Instant Water Cooler Making Guide | DIY Cooling Solution
DIY air cooler/air conditioner fan/homemade air cooler/table fan cooling Idea/#aircooler #tdmade
How to make an instant cooler
Комментарии
 0:11:17
0:11:17
 0:07:53
0:07:53
 0:00:48
0:00:48
 0:11:17
0:11:17
 0:00:23
0:00:23
 0:11:46
0:11:46
 0:00:56
0:00:56
 0:00:56
0:00:56
 0:00:25
0:00:25
 0:00:44
0:00:44
 0:00:32
0:00:32
 0:21:32
0:21:32
 0:00:12
0:00:12
 0:11:01
0:11:01
 0:09:00
0:09:00
 0:15:13
0:15:13
 0:06:54
0:06:54
 0:11:12
0:11:12
 0:00:40
0:00:40
 0:14:37
0:14:37
 0:00:20
0:00:20
 0:00:59
0:00:59
 0:01:01
0:01:01
 0:03:47
0:03:47