filmov
tv
R3.1.15 Identify an appropriate indicator for a titration [HL IB Chemistry]

Показать описание
When [In-] = [HIn] then the indicator is at colour change. This should be at equivalence point for the titration. The pH of the equivalence point = pka of the indicator.
Crazy Fibonacci Retracement Trick
How TO IDENTIFY Breakout #ChartPatterns Candlestick | Stock | Market | Forex | crypto #Shorts
What's The Secret Strategy For Trading?
The Easiest Scalping Strategy
3 Tips To Defend Like A Pro Player In FIFA 23
What Are Pivot Points? - Trade Like a BANK
How to use pivot points to increase your profits shorts | Trading shorts
Resistor clour code.#short#viralshort#youtubeshort
🔥 #rsi RSI strategy 300+ points #st #shortvideo #shorts #ytshorts #nifty50 #rsistrategy #intraday
🔥R15 Abs Saved my Life 🔥
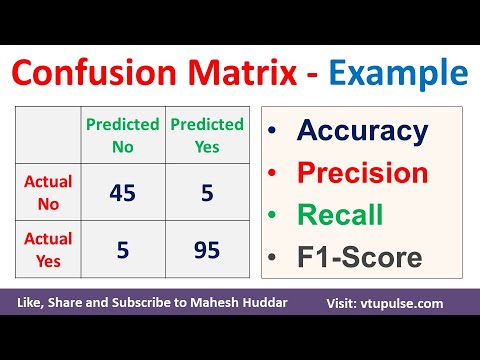
Confusion Matrix Solved Example Accuracy Precision Recall F1 Score Prevalence by Mahesh Huddar
HOW TO CHECK MOTORCYCLE OIL LEVEL
this happens when you use 3200mhz ram in 2133 mhz supported motherboard
Lipo 4s under volt super explosion
Sounds like a Rod Knock noise, but it's NOT. Get a second opinion for the Repair
Color Settings You NEED to Change (Valorant Tips)
No more PS5 controller stick drift?..
eh awas kamu kamu kan gk punya hp #shorts
Stock Screener For Intraday Trading | 3-4% Return Daily💸💸
🔴 High Accuracy SCALPING & INTRADAY Price Action Trading with PIVOT POINTS Support-Resistance
3 Tips To Score Like A Pro In FIFA 23
Bike Indicators Fault Finding
What engineering students actually do in labs 💀 #electronics #arduino #engineering
Best Electronic Project with BC547 Transistor #shorts
Комментарии
 0:00:59
0:00:59
 0:00:16
0:00:16
 0:00:33
0:00:33
 0:01:00
0:01:00
 0:00:35
0:00:35
 0:04:33
0:04:33
 0:00:58
0:00:58
 0:00:16
0:00:16
 0:01:00
0:01:00
 0:00:19
0:00:19
 0:05:50
0:05:50
 0:01:00
0:01:00
 0:00:55
0:00:55
 0:00:12
0:00:12
 0:00:16
0:00:16
 0:00:23
0:00:23
 0:00:14
0:00:14
 0:00:48
0:00:48
 0:00:47
0:00:47
 0:13:27
0:13:27
 0:00:34
0:00:34
 0:02:00
0:02:00
 0:00:22
0:00:22
 0:00:51
0:00:51