filmov
tv
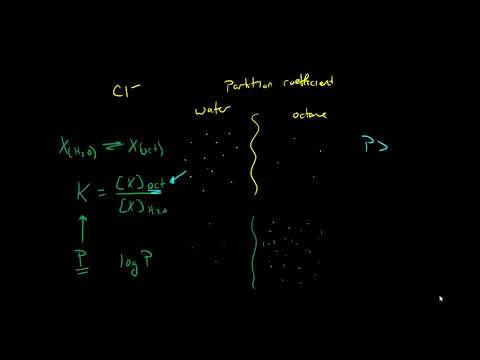
Partition coefficients

Показать описание
A lecture for the University of Lincoln on partition coefficients and why they are important in drug absorption. Both LogP and LogD are explained and their relationship to pH.
A textbook based on this YouTube channel is now available. Clinical Pharmacokinetics From The Beginning (a practical approach) can be found as an e-book or paperback from Amazon.
Note that the book does not cover partition coefficients but will be of interest to those requiring knowledge of basic pharmacokinetics.
A textbook based on this YouTube channel is now available. Clinical Pharmacokinetics From The Beginning (a practical approach) can be found as an e-book or paperback from Amazon.
Note that the book does not cover partition coefficients but will be of interest to those requiring knowledge of basic pharmacokinetics.
What is a Partition Coefficient?
Partition Coefficient
Partition Coefficient (Kd)
Octanol-Water Partition Coefficient and Medication Absorption
Topic 4.3-Calculation on Partition Coefficient, Kpc
Equilibria 03: Partition coefficient or distribution law: A level chemistry: Class 12
Igneous Petrology Series: Lesson 2 - Element compatibility & partition coefficients
What is Partition Coefficients ?
Calcul très simple
Blood:Gas Partition Coefficients
Topic 4.3-Introduction on Partition Coefficient, Kpc
Partition coefficients
Oil:Gas Partition Coefficients
Partition Coefficients 1: Notation
Mono Craters Example 1: Introduction to partition coefficients and set up
Solubility | Partition Coefficient | physicochemical properties (P-2) | L-4, U-1 | Medicinal Chem 1
This is Where You Go WRONG with Partition Coefficients! | A Level | Conquer Chemistry
Partition Coefficient | Definition| Pharmacy Dictionary @PharmacistTayyebOfficial
Partitioning Between Liquid Phases
Partition Coefficient & Lipophilicity (FTM 1)
Lec 6 Blood Gas Partition Coefficient mp4
Partition coefficient and element compatibility
Solubility products, the common ion effect and partition coefficients (Equilibria #8)
The Partitioning Based Laboratory For Organic Pollutants
Комментарии
 0:09:51
0:09:51
 0:04:29
0:04:29
 0:04:45
0:04:45
 0:04:54
0:04:54
 0:13:25
0:13:25
 0:21:07
0:21:07
 0:07:30
0:07:30
 0:00:32
0:00:32
 0:00:09
0:00:09
 0:09:11
0:09:11
 0:08:30
0:08:30
 0:21:02
0:21:02
 0:08:02
0:08:02
 0:03:13
0:03:13
 0:04:37
0:04:37
 0:12:31
0:12:31
 0:00:57
0:00:57
 0:06:48
0:06:48
 0:05:57
0:05:57
 0:16:32
0:16:32
 0:03:31
0:03:31
 0:04:25
0:04:25
 0:28:45
0:28:45
 0:49:47
0:49:47