filmov
tv
Calculating Isotope Abundance using Atomic Mass

Показать описание
This is a more challenging problem using atomic mass. I show you haw to calculate the percentages of the isotopes of an element using the masses of the isotopes and the atomic mass from the periodic table.
Atomic Mass: How to Calculate Isotope Abundance
Calculating Isotope Abundance using Atomic Mass
How To Find The Percent Abundance of Each Isotope - Chemistry
How to Solve for Percent Abundance of Isotopes Examples, Practice Problems, Step by Step Explanation
How to Calculate Isotope Abundance Using Atomic Mass!
ALEKS: Finding atomic mass from isotope mass and natural abundance
Calculating the Mass for Isotopes with Natural Abundance
ALEKS: Finding isotope mass or natural abundance from atomic mass
What are Isotopes?
Isotopes, Percent Abundance, Atomic Mass | How to Pass Chemistry
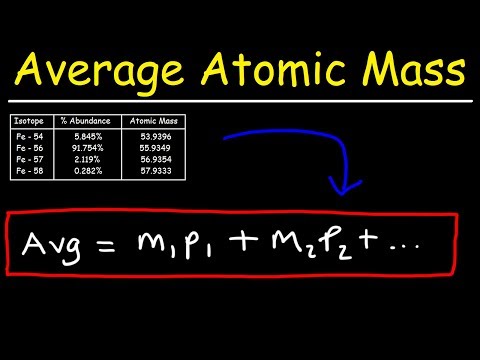
How To Calculate The Average Atomic Mass
Chemistry A level Relative atomic mass difficult question solved pass your exams @letsgettothemarks
How to calculate percentage abundance of each isotope ?
Practice Problem: Isotopic Abundance and Atomic Mass
Finding isotope mass or natural abundance from atomic mass
Isotopic Abundance (Example)
Finding Percent Abundance (3 Isotopes)
Calculating the abundance of isotopes using mass spectra
CALCULATING % ABUNDANCE OF ISOTOPES - SIMPLE 'TUG OF WAR' METHOD
Calculating Isotope Percent Abundance
How to Calculate Atomic Mass Practice Problems
How To Calculate The Average Atomic Mass | Isotope Abundance
Chemistry Help: How to Calculate the Percent Abundance of an Isotope
What is the percentage abundance of each isotope of Li
Комментарии
 0:11:49
0:11:49
 0:03:50
0:03:50
 0:10:18
0:10:18
 0:05:12
0:05:12
 0:08:46
0:08:46
 0:04:29
0:04:29
 0:07:06
0:07:06
 0:08:05
0:08:05
 0:18:51
0:18:51
 0:12:15
0:12:15
 0:07:19
0:07:19
 0:05:54
0:05:54
 0:07:20
0:07:20
 0:03:44
0:03:44
 0:08:55
0:08:55
 0:02:29
0:02:29
 0:02:28
0:02:28
 0:09:05
0:09:05
 0:04:17
0:04:17
 0:17:28
0:17:28
 0:06:11
0:06:11
 0:02:36
0:02:36
 0:01:29
0:01:29
 0:01:37
0:01:37