filmov
tv
Heterogenous vs Homogenous (Definitions, Examples, & Practice)

Показать описание
When we discuss mixtures we can broadly classify them as heterogenous or homogenous. This is common question on chemistry quizzes and tests where you are given a mixture, for example salt water, and asked to determine whether is a heterogenous or homogenous mixture.
The general definition is:
Heterogenous: Not uniform (unevenly distributed).
Examples: rocks in mud, salad, nails in sand
Homogenous: Uniform composition (same throughout).
Examples: salt water, smoke, alloys
Note that you can have a mixture, like rock, water, and sugar that has hetero and homo components. In this case the rocks and sugar water are a heterogenous mixture. If we look just at the sugar dissolved in the water, this has a uniform/smooth composition and is therefore a homogenous solution.
We can separate both heterogenous and homogenous mixtures using their physicals properties. For example filtration, distillation, sorting based on size, or gravitational sorting based on density.
The general definition is:
Heterogenous: Not uniform (unevenly distributed).
Examples: rocks in mud, salad, nails in sand
Homogenous: Uniform composition (same throughout).
Examples: salt water, smoke, alloys
Note that you can have a mixture, like rock, water, and sugar that has hetero and homo components. In this case the rocks and sugar water are a heterogenous mixture. If we look just at the sugar dissolved in the water, this has a uniform/smooth composition and is therefore a homogenous solution.
We can separate both heterogenous and homogenous mixtures using their physicals properties. For example filtration, distillation, sorting based on size, or gravitational sorting based on density.
Heterogenous vs Homogenous (Definitions, Examples, & Practice)
Homogeneous and Heterogeneous Mixtures Examples, Classification of Matter, Chemistry
Homogeneous and Heterogeneous Mixture | Chemistry
10 Examples of Homogeneous Mixtures and Heterogeneous Mixtures
Science Quiz: Homogeneous or Heterogeneous Mixtures - Part 1 | ANY 10
Heterogeneous and Homogeneous Mixtures in Chemistry
Example of Heterogenous and Homogeneous
Homogenous vs Heterogenous Grade 10
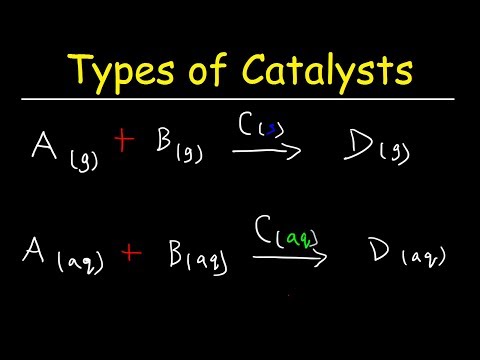
Homogeneous vs Heterogeneous Catalysts - Basic Introduction
EXAMPLES OF HOMOGENEOUS AND HETEROGENEOUS!
Homogeneous Vs. Heterogeneous Mixture
Heterogeneous Mixture And Homogeneous Mixture
homogeneous and heterogeneous mixture examples
HETEROGENEOUS AND HOMOGENEOUS MIXTURES!
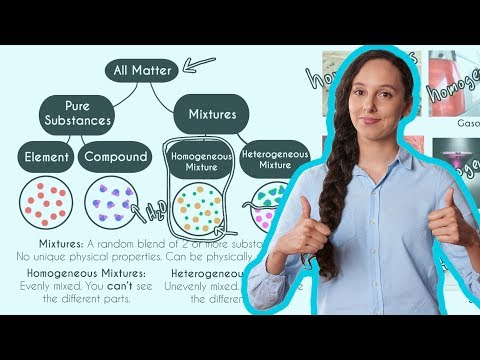
Pure Substances and Mixtures | Science for Kids
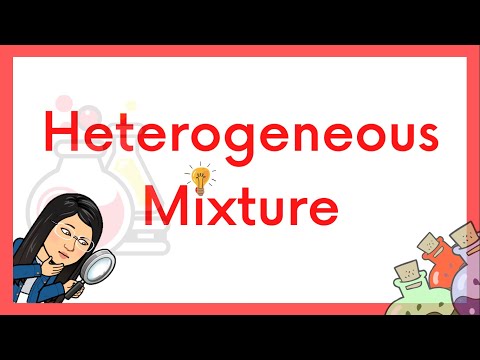
Heterogeneous Mixture | Definition & Examples
Difference between Homogeneous mixture and Heterogeneous mixture
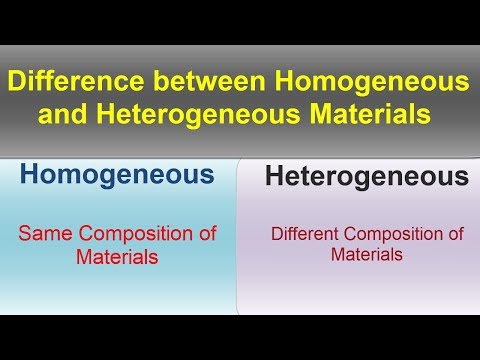
Difference between Homogeneous and Heterogeneous Material
Homogeneous and Heterogeneous Mixtures!
HETEROGENEOUS and HOMOGENEOUS MIXTURE: Their difference| Simple Experiment
HETEROGENEOUS mixture l suspension l colloid l immersion l MELC l S6MT-Ia-c-1 l TEACHER Essentials
Homogeneous vs Heterogeneous Mixture
Heterogeneous Mixture #shorts
Homogeneous and heterogeneous mixture
Комментарии
 0:03:35
0:03:35
 0:05:50
0:05:50
 0:05:01
0:05:01
 0:03:41
0:03:41
 0:03:22
0:03:22
 0:11:39
0:11:39
 0:03:03
0:03:03
 0:04:29
0:04:29
 0:01:34
0:01:34
 0:00:06
0:00:06
 0:06:59
0:06:59
 0:02:16
0:02:16
 0:00:07
0:00:07
 0:00:11
0:00:11
 0:04:39
0:04:39
 0:04:03
0:04:03
 0:04:38
0:04:38
 0:02:45
0:02:45
 0:07:39
0:07:39
 0:02:24
0:02:24
 0:08:28
0:08:28
 0:00:21
0:00:21
 0:00:16
0:00:16
 0:00:16
0:00:16