filmov
tv
Production of green hydrogen through water electrolysis - how does it work?

Показать описание
Green hydrogen is called the fuel of the future as it has the ability to power the hard to electrify sectors like industry, transportation and buildings,
contributing around 65% of yearly greenhouse gas emission in Europe.
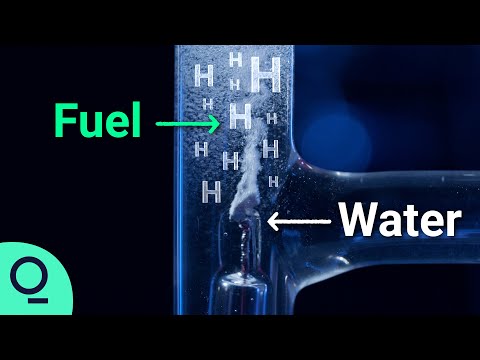
Hydrogen can be produced through a process called water-electrolysis. This process takes place in a unit called an electrolyser. Electrolysers split water into hydrogen and oxygen molecules using electricity, which then provokes a chemical reaction. Direct current at an anode and a cathode splits water into oxygen and hydrogen.
The produced hydrogen is then ready for use in direct application like transport, industry feedstock and steel production.
Indirect, power-to-x applications like, e-fuels, fertilizers, and replacement for natural gas. And lastly grid stabilization and storage.
If the electricity comes from renewable energy sources, such as solar and wind, the hydrogen is completely CO2-free and truly green.
contributing around 65% of yearly greenhouse gas emission in Europe.
Hydrogen can be produced through a process called water-electrolysis. This process takes place in a unit called an electrolyser. Electrolysers split water into hydrogen and oxygen molecules using electricity, which then provokes a chemical reaction. Direct current at an anode and a cathode splits water into oxygen and hydrogen.
The produced hydrogen is then ready for use in direct application like transport, industry feedstock and steel production.
Indirect, power-to-x applications like, e-fuels, fertilizers, and replacement for natural gas. And lastly grid stabilization and storage.
If the electricity comes from renewable energy sources, such as solar and wind, the hydrogen is completely CO2-free and truly green.
Green hydrogen production
How Green Hydrogen Could End The Fossil Fuel Era | Vaitea Cowan | TED
Green Hydrogen - Production, Storage and Transportation
What is Green Hydrogen?
Production of green hydrogen through water electrolysis - how does it work?
What Is Green Hydrogen And Will It Power The Future?
REVOLVE | How To Produce Green Hydrogen
PEM electrolysis at Bosch | Scaling production of green hydrogen
Hydrogen with Carbon Management 101
Can hydrogen powered plants turn steel production 'green'? | DW News
Green Hydrogen Systems Electrolyser Tour
With this technology you can produce your own Hydrogen at home for free
World's Largest Green Hydrogen Projects
Green Hydrogen Systems: Electrolysis Technology
Electrolysis: Producing hydrogen from water
Wastewater to Green Hydrogen: Transforming Waste into Renewable Energy
Lecture 6: Green Hydrogen Production, Biomass Gasification
World largest green hydrogen plant
How do you make GREEN HYDROGEN? | The Secrets Behind PEM Electrolysis
Can hydrogen help the world reach net zero? | FT Film
15 Ton Per Day Green Hydrogen Plant Update
The truth about hydrogen
How Cheap Hydrogen Could Become the Next Clean Fuel
Sustainable hydrogen made possible with a new catalyst
Комментарии
 0:02:16
0:02:16
 0:09:15
0:09:15
 0:19:47
0:19:47
 0:02:11
0:02:11
 0:01:08
0:01:08
 0:15:26
0:15:26
 0:01:01
0:01:01
 0:03:51
0:03:51
 0:02:19
0:02:19
 0:08:07
0:08:07
 0:15:10
0:15:10
 0:04:10
0:04:10
 0:09:47
0:09:47
 0:06:44
0:06:44
 0:00:54
0:00:54
 0:05:23
0:05:23
 0:03:08
0:03:08
 0:10:54
0:10:54
 0:01:35
0:01:35
 0:24:46
0:24:46
 0:03:25
0:03:25
 0:12:08
0:12:08
 0:12:32
0:12:32
 0:00:42
0:00:42